Syeed Aalishan Fatima1*, Mahrukh2, Majid Jehangir3
1Senior Resident, Department of Diagnostic and Interventional Radiology, Government Medical College Srinagar Jammu and Kashmir, India
2Junior Resident, Department of Diagnostic and Interventional Radiology, Government Medical College Srinagar Jammu and Kashmir, India
3Professor and Head of Department, Department of Diagnostic and Interventional Radiology, Government Medical College Srinagar Jammu and Kashmir, India
*Correspondence author: Syeed Aalishan Fatima, Senior Resident, Department of Diagnostic and Interventional Radiology, Government Medical College Srinagar Jammu and Kashmir, India; Email: [email protected]
Published Date: 07-11-2024
Copyright© 2024 by Fatima SA, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Stroke is the second leading cause of mortality and morbidity worldwide, with spontaneous Intracranial Hemorrhage (ICH) accounting for 9%-27% of all strokes. The study evaluates clinical and NCCT markers to predict early Hematoma Expansion (HE) in patients with Intracranial Hemorrhage (ICH), focusing on directing management objectives.
Methods: We prospectively evaluated 96 patients with spontaneous ICH who underwent a baseline NCCT within four hours of admission, followed by a follow-up scan within six hours or at the time of clinical deterioration, whichever was earlier. Hematoma volumes were determined using baseline and follow-up CT images and imaging characteristics that predicted HE were evaluated. A grading system score was created to predict HE.
Results: Of the ninety-six patients studied, 29 displayed black hole signs, 31 had island signs and 22 had swirl signs on baseline NCCT. On follow-up scans, the total incidence of HE in ICH patients was 53 out of 96 (55.2%). The average baseline hematoma volume in HE patients was 44.1 ml, compared to 12.2 ml in non-hematoma expansion patients. Of the 53 patients with HE, 29 had black hole sign, 16 had swirl sign and 28 had island sign. A higher grading system score (P < 0.001) was associated with a higher likelihood of HE.
Keywords: Intracranial Hemorrhage (ICH); Non-Contrast Computed Tomography (NCCT); Hematoma Expansion (HE); Intraventricular Hemorrhage (IVH) Island Sign; Swirl Sign
Introduction
Stroke is the second leading cause of mortality and morbidity worldwide, following ischemic heart disease [1]. Spontaneous ICH accounts for 9%-27% of all strokes worldwide [2]. Furthermore, intracranial hemorrhage has a greater mortality and morbidity compared to ischemic stroke [3].
Recently many indicators based on the initial NCCT scan (blend sign, black hole sign, swirl sign and island sign) have been demonstrated to predict early hematoma expansion and thus identify high-risk patients [4,5].
Because of the high morbidity and mortality rates following Intra Cerebral Hemorrhage (ICH), identifying such patients would be valuable in directing the management course and treatment objectives. Therefore, we undertook the study to evaluate various clinical and NCCT markers on the initial scan to predict early hematoma expansion and outcome in ICH patients.
Material and Methods
Patient Selection
The prospective study was conducted over a 26-month period after receiving approval from the institutional ethics committee. 115 adult patients with spontaneous ICH were initially included in the study. Ten critical patients were admitted to intensive care units and nine patients could not be reached for follow-up and were therefore excluded from the study. 96 adult (>18 YEARS) patients with a history of acute cerebrovascular stroke who were diagnosed with spontaneous ICH based on a non-contrast CT performed within 4 hours of admission and who underwent a follow-up NCCT scan within a 6-hour period or at the time of clinical deteriotion, whichever is earlier and completed the follow-up, were enrolled in the study (Fig. 1).
Secondary ICH caused by a brain tumor, trauma, or vascular malformation; hemorrhagic transformation of a cerebral infarct; ischemic stroke; or venous thrombosis and patients with coagulopathy and/or on antiplatelet and anticoagulant medications were excluded.
A detailed medical history was obtained for all patients, with a focus on hypertension, diabetes, smoking, anticoagulant and antiplatelet medication, illicit drug use and antihypertensive medicines. A clinical examination was performed, with an emphasis on neurological evaluation. The patients underwent routine blood tests such as complete blood count, a kidney function test, electrolytes, random blood sugar.
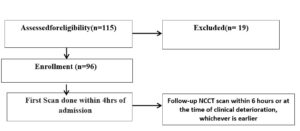
Figure 1: Flowchart.
Image Acquisition and Analysis
The first scan was performed within four hours of admission and a follow-up NCCT was performed within 6 hour after the initial CT or earlier if clinical deterioration occurred. All CT scans were done using a 16-slice Siemens Somatom Emotion Multidetector CT ( Erlangen, Germany), 100-120 kVp and 240 mAs using automatic exposure control, a CTDI volume of 38.69 mGy, a DLP of 745.86 mGycm, a slice thickness of 5.0 mm and a beam pitch of 0.5. The images were taken in 5 mm slices and reconstructed into 1.5 mm. The images were seen with window settings of 80 HU and centering of 30 HU. Two radiologists reviewed CT images in a satellite room on an Apple Mac Workstaion, with consensus decisions made by a third expert radiologist with 21 years of experience in case of conflict.The radiologists assessed hematomas in various locations, including supratentorial, infratentorial, lobar and basal ganglia. They assessed perihematomal edema, mass effect, midline shift, intraventricular hemorrhage and subarachnoid hemorrhage. They evaluated the images on a workstation for the following NCCT features and markers.
Hematoma Volume
Hemorrhage volumes were measured using a simplified formula for the volume of an ellipsoid, ABC/26. The CT slice with the largest region of hemorrhage was selected for the bedside ABC/2 technique. The maximum diameter (A) of the hemorrhage on this slice was measured. The largest diameter 90 degrees to A on the same slice was measured next (B). Finally, the approximate number of 10-mm slices displaying the ICH was determined (C). C was computed by comparing each CT slice with hemorrhage to the CT slice with the most hemorrhage on the image (D).
The swirl sign was characterized as a region(s) of hypoattenuation or isoattenuation inside the hyperattenuated ICH (in comparison to brain parenchyma attenuation). The hypoattenuation or isoattenuation regions might be spherical, streaky, or irregular in form [7-9].
The black hole sign was described as a circular or oval-shaped region of hypoattenuation that is surrounded by the hyperattenuating hematoma and has no relationship to the nearby brain parenchyma. It exhibits variable density, implying bleeding at several time points and thus predictive of hematoma extension. The density difference between the hypo-attenuated and hyper-attenuated hematomas is stated as at least 28 Hounsfield units [8,10].
The island sign was characterized as three or more distributed small oval or round hematomas that are all independent from the main hematoma or four or more small bubble-like or sprout-like but not lobulated hematomas that might be connected with the main hemorrhage [11].
Hematoma Expansion (HE) was defined as a relative increase in hematoma volume of more than 33% compared to the initial volume observed on CT or an absolute increase in hematoma volume of more than 12.5% over the baseline ICH volume [10,12].
Statistical Analysis
Microsoft Excel was used to enter the data. Continuous variables were described using medians and Interquartile Ranges (IQRs) or the mean Standard Deviation (SD), while categorical variables were described using percentages (%). The T-test was used to compare continuous data, while the chi-square test was used to evaluate categorical variables. Analyses of multivariate and univariate logistic regression were utilized to evaluate the factors connected to hematoma growth. Statistics were considered significant at p< 0.05.
Results
Demographic, Clinical and Base Line Characteristics
The study included 96 patients with spontaneous ICH; The study included 60 males (62.5%) and 36 females (37.5%). (Male to Female Ratio: 1.7:1). The mean age of the patients was 60.2 ± 10.85 years (range: 34-88 years). Hypertension was the most prevalent risk factor in 75(78.1%) followed by diabetes mellitus in 37 patients (38.5%); the frequency of other risk factors was lower (Table 1).
Among 96 patients, the baseline hematoma occurred in the capsuloganglionic region in 50 (52.1%), the cerebral lobes in 35 (36.5%) and infratentorial in 11 (11.5%). On admission, the volume of baseline hematoma was < 30 ml in 57 (59.4%) and >30 ml in 39 (40.6%). Intraventricular hemorrhage was present in 54(56.3%) and subarachnoid hemorrhage in 3 (3.1%) (Table 1). The mean baseline hematoma volume in hematoma expansion patients was 44.1 ml, versus 12.2 ml in non-hematoma expansion patients (p<0.001). Box plot graph depicting the relationship between baseline hematoma volume and hematoma expansion (Fig. 1). Intraventricular hemorrhage was seen in 41 (77.4%) of HE patients and 13 (30.2%) of non-HE patients (p<0.001). Subarachnoid hemorrhage was observed in 2 (3.8%) with hematoma expansion compared with 1 (2.3%) without hematoma expansion (Table 2).
The Three NCCT Markers’ Prevalence and Features
Of the 96 ICH patients, the island sign, swirl sign and black hole sign were seen in 31 (32.3%), 22, (22.9%), 36 (8.70%) and 29 (30.2%), respectively on the baseline NCCT scan. On follow-up scans, the total incidence of HE in patients with ICH was 53 of 96 (55.2%) patients.Out of the 53 patients who had HE, 28 (52.8%) had the island sign (p<0.05%). 21 (39.6%) (p<0.05%) had the black hole sign and 16 (30.2%) had the swirl sign (p<0.05%)(p<0.005). The study found that patients with island signs had a mean baseline hematoma volume of 40.9 ±27.03 mL(p<0.005). Patients with swirl signs had a mean baseline volume of 37.9.9 ± 21.62 mL (p<0.005) and those with black hole signs had a mean baseline volume of 35.8 ± 21.98 mL (p<0.005). Box plot graphs depicting the relationship between baseline hematoma volume and Island sign, Swirl sign and Black hole sign (Fig. 2-5s). Of the three NCCT markers evaluated in this study, island sign had the highest sensitivity (52.8%) and specificity (93.1%). The accuracy of the three NCCT markers varied, with the swirl sign having the lowest accuracy at 55.2% and the island sign having a relatively high accuracy of up to 70.8% (Table 3).
Devising and Validation of the Grading System
A multivariate logistic regression( Table 4) found that the Baseline hematoma volume ≥ 30 ml (OR, 1.9; 95% CI,1.41-2.74;P=0.039), IVH (OR, 4.5; 95% CI,2.79-6.82;P=0.008), Island sign (OR, 13.7; 95% CI,10.15-16.37;P=<0.001), Swirl sign (OR, 5.2; 95% CI, 3.72-6.53;P=<0.001) and Black hole sign (OR, 9.4; 95% CI,7.4-11.62;P=<0.001) were all independent significant predictors of HE. The parameters from multivariable regression in were used to create a grading system (Table 5), which included imaging indicators (island sign, swirl sign, blackhole sign), baseline hematoma volume and IVH extension. The grading system was utilized to assess patients’ ability to predict hematoma expansion, with higher scores indicating a higher likelihood of expansion. The likelihood of HE was 7.4% (2/27) for a score of 0, 37.5% (6/16) for a score of 1, 75% (15/20) for a score of 2, 85.7% (12/14) for a score of 3 and 93.3% (14/15) for a score of 4. With a score of 5, the chance of HE was 100% (4/4) (Table 6).
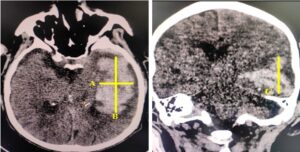
Figure 2: Calculating volume of hematoma in NCCT scan brain in intracranial bleed using ABC/2=volume in ml. A = maximum length in cm. B= maximum width in cm perpendicular to the line of length in the same CT slice. C= The longest cranio-caudal measurement on the coronal section as C.
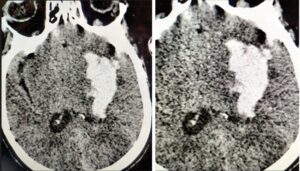
Figure 3: A: A 59-year-old man presented with a sudden onset of right-sided weakness. On Admission Axial Noncontrast Computed Tomography (NCCT) image performed 1.5 hours after hospital admission showed a left basal ganglia bleed with a swirl sign; B: Follow-up Axial Non-Contrast Computed Tomography (NCCT) 8 hours later showed a significant hematoma expansion.

Figure 4: A: 68-year-old woman presented with a sudden onset of aphasia, right-sided weakness. On admission a axial noncontrast computed tomography (NCCT) image performed 3 hours after hospital admission showed left basal hematoma with a black hole sign; B: Follow-up axial non-contrast computed tomography (NCCT) 7 hours later showed a significant hematoma expansion with intra-ventricular extension.
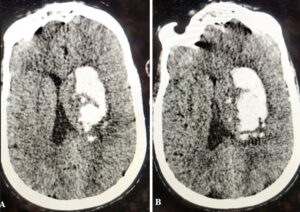
Figure 5: A: 66-year-old man presented with a sudden onset of left-sided weakness. On admission axial non-contrast computed tomography image performed 2 hours after hospital admission showed a lobar hematoma with an island sign (arrows); B: Follow-up CT 6 hours later, it had significant hematoma expansion.
|
Gender |
Number (n) |
Percentage (%) |
|
Male |
60 |
62.5 |
|
Female |
36 |
37.5 |
|
Total |
96 |
100 |
|
Male:Female=1.7:1 |
||
|
Comorbidity |
|
|
|
Hypertension |
75 |
78.1 |
|
Diabetes |
37 |
38.5 |
|
Chronic kidney disease |
33 |
35.1 |
|
Smoking |
23 |
24 |
|
Dyslipidemia |
19 |
19.8 |
|
Alcohol intake |
3 |
3.1 |
|
Parameter |
||
|
Hematoma location |
Capsuloganglionic |
50 |
52.1 |
|
Lobar |
35 |
36.5 |
|
|
Infratentorial |
11 |
11.5 |
|
|
IVH |
Present |
54 |
56.3 |
|
Absent |
42 |
43.8 |
|
|
SAH |
Present |
3 |
3.1 |
|
Absent |
93 |
96.9 |
|
|
Baseline hematoma volume |
< 30 ml |
57 |
59.4 |
|
≥ 30 ml |
39 |
40.6 |
|
|
Midline shift |
Present |
74 |
77.1 |
|
Absent |
22 |
22.9 |
|
|
Island sign |
Present |
31 |
32.3 |
|
Absent |
65 |
67.7 |
|
|
Swirl sign |
Present |
22 |
22.9 |
|
Absent |
74 |
77.1 |
|
|
Black hole sign |
Present |
29 |
30.2 |
|
Absent |
67 |
69.8 |
Table 1: The baseline demographics, clinical, radiological characteristics.
|
Variable |
HE |
No HE |
P-value |
|
Baseline hematoma volume (ml); Mean±SD |
44.1±26.34 |
12.2±16.32 |
<0.001* |
|
IVH, n (%) |
41 (77.4) |
13 (30.2) |
<0.001* |
|
SAH, n (%) |
2 (3.8) |
1 (2.3) |
0.685 |
|
Island sign, n (%) |
28 (52.8) |
3 (7.0) |
<0.001* |
|
Swirl sign, n (%) |
16 (30.2) |
6 (14.0) |
0.049* |
|
Black hole sign, n (%) |
21 (39.6) |
8 (18.6) |
0.026* |
Table 2: Baseline demographic and NCCT imaging characteristics of study population with and without hematoma expansion.
|
Variable |
IS/SS/BS (+) |
IS/SS/BS (-) |
P-value |
|
Imaging characteristics as per Island Sign |
|||
|
Baseline hematoma volume (ml); Mean±SD |
40.9±27.03 |
24.5±26.08 |
0.005* |
|
HE, n (%) |
28 (90.3) |
25 (38.5) |
<0.001* |
|
Imaging characteristics as per Swirl Sign |
|||
|
Baseline hematoma volume (ml); Mean±SD |
37.9±21.62 |
25.4±23.97 |
0.031* |
|
HE, n (%) |
16 (72.7) |
37 (50.0) |
0.049* |
|
Imaging characteristics as per Black hole Sign |
|||
|
Baseline hematoma volume(ml); Mean±SD |
35.8±21.98 |
26.1±18.85 |
0.045* |
|
HE, n (%) |
21 (72.4) |
32 (47.8) |
0.026* |
|
IS: Island Sign; Swirl Sign, Black hole Sign *Statistically significant Difference (P-value<0.05) |
|||
Table 3: Imaging characteristics as per Island sign, swirl sign, black hole sign.
|
Parameter |
OR |
95% CI |
P-value |
|
Baseline hematoma volume ≥ 30 ml |
1.9 |
1.41-2.74 |
0.039* |
|
IVH |
4.5 |
2.79-6.82 |
0.008* |
|
Island sign |
13.7 |
10.15-16.37 |
<0.001* |
|
Swirl sign |
5.2 |
3.72-6.53 |
<0.001* |
|
Blackhole sign |
9.4 |
7.4-11.62 |
<0.001* |
|
OR: Odds Ratio; CI: Confidence Interval; *Statistically Significant (P value<0.05) |
|||
Table 4: Multivariate logistic regression depicting predictors for hematoma expansion.
|
Component |
Points |
|
Baseline hematoma volume (ml) |
|
|
≥ 30 |
1 |
|
< 30 |
0 |
|
IVH |
|
|
Present |
1 |
|
Absent |
0 |
|
Island sign |
|
|
Present |
1 |
|
Absent |
0 |
|
IVH |
|
|
Present |
1 |
|
Absent |
0 |
Table 5: Summary of the HE prediction grading system.
|
Total score |
No. of Patients |
HE |
Risk of HE (%) |
|
0 |
27 |
2 |
7.4 |
|
1 |
16 |
6 |
37.5 |
|
2 |
20 |
15 |
75.0 |
|
3 |
14 |
12 |
85.7 |
|
4 |
15 |
14 |
93.3 |
|
5 |
4 |
4 |
100 |
Table 6: Risk of Hematoma Expansion (HE) according to the prediction grading system.
Discussion
A leading cause of morbidity and mortality globally is still spontaneous Intracerebral Hemorrhage (ICH) [13]. One interesting treatment target is Hematoma Expansion (HE), which is also a potentially modifiable predictor of outcome [14,15]. A number of NCCT imaging markers have been proposed recently for the prediction of hematoma expansion, including the blend sign, the black hole sign and CT hypodensities [16-18].
In our study, Spontaneous Intracerebral Hemorrhage (SICH) was more common in the 61-70-year-old age group, with hypertension (78.1%) and diabetes (38.5 %) being the most common comorbidities. These findings are in line with studies by Romero JM, et al., which found that 47% of the study population had diabetes and by Morotti A, et al., who reported hypertension in 81.7%, 79% and 71% of the three cohorts in his study [19,20].
The capsuloganglionic region is the most involved site, with 55.2% of patients showing Hematoma Expansion (HE). The overall incidence of HE in our study patients with ICH was 55.2%, somewhat higher than reported in recent studies by Yongwei Huang, et al. [21]. This disparity could be explained by three factors: first, differences in study group composition, second, various sample sizes and variables linked with HE and NCCT indications. Third, all of the patients enrolled presented within 6 hours after admission, rather than 24 hours as in previous studies.
In our study, we observed various characteristics that have been related to early hematoma expansion on NCCT. Consistent with previous observations [22-24]. We found that initial baseline hematoma volume is an independent predictor of early hematoma expansion (P<0.001). The mean baseline hematoma volume in patients with HE was 44.1 ml, versus 12.2 ml in patients without HE. (p<0.001). A large hematoma at baseline CT scan may exacerbate the effect of vascular shearing, leading to hematoma expansion [25].
In this study, we studied the association between intraventricular hemorrhage and early hematoma expansion).Intraventricular hemorrhage is not rare in ICH patients. It has been reported in 30-50% of people with ICH [26,27]. Additionally, intraventricular hemorrhage is a strong independent predictor of poor outcomes in ICH patients [28,29].We identified an significant association between the presence of intraventricular hemorrhage on CT scan and early hematoma expansion. IVH observed in 77.4% of patients with HE compared to 30.2% of patients without HE(p<0.001).
Our study found that imaging markers on Non-enhanced CT (NCCT), such as the island sign, swirl sign and blackhole sign, independently predict early hematoma expansion in patients with intracerebral hemorrhage (p<0.005).The study found three NCCT markers as predictors of early HE, with high specificity and moderate accuracy, which could aid in determining the best therapeutic strategy for ICH patients.These NCCT markers are linked with early hematoma expansion and have been verified by various studies [17,30]. Every imaging marker in the study has shown an effective ability to predict HE. The island sign, blackhole and swirl signs can all predict early HE, in that order the island sign was more accurate in predicting hematoma expansion (p<0.001).
We also found that patients with these imaging markers have a greater hematoma volume than those without it (p< 0.005),which is consistent with studies by Huang Y, et al. [21]. Baseline hematoma volume > 30 ml and imaging markers on the baseline CT scan (p< 0.001 for all) were the significantly associated with early HE in a multivariate logistic regression analysisas demonstrated by Huang Y, et al. A grading system was developed using multivariable regression variables such as imaging markers (island sign, swirl sign, blackhole sign), baseline hematoma volume and IVH extension [21]. The grading system was utilized for assessing patients in order to assess its ability to predict HE.
The grading method was used to predict HE in patients, with a higher score (p< 0.001) indicating a higher likelihood of HE. The prediction grading system results are comparable to those from previous studies. Brouwers, et al., developed a 9-point score to predict HE based on four parameters: the presence of the spot sign, warfarin use, the time to the initial CT (> 6 h or ≤ 6 h) and baseline ICH volume (< 30 mL, 30- 60 mL or > 60 mL) [31]. A higher score resulted in a better ability to predict HE in their study. In 2015, Wang, et al., refined the 9-point score by including baseline ICH volume (≤ 10 mL,10-20 mL or > 20 mL) and the time to the initial CT (≤ 1 h, 1-2 h, 2-3 h, 3-4 h, 4-5 h and > 5 h) [32]. Based on the 9-point score, Wang, et al., proposed a new 24-point score (BRAIN) and they added two novel parameters: IVH extension and recurrent ICH [32]. Since NCCT is so widely used, practically any medical facility may easily ascertain the characteristics required to calculate a score using this grading system.
Conclusion
In conclusion, our study demonstrated that the island sign, blackhole sign, swirl sign, IVH and baseline hematoma volume of more than 30 ml all accurately predicted early hematoma expansion. The island sign predicts early HE, followed by the blackhole and swirl signs in that order. Early HE was most strongly predicted by baseline hematoma volume ≥ 30 ml, IVH and imaging markers on the baseline CT scan (all p< 0.001). The study used a 5-point prediction grading system, with higher scores indicating a higher likelihood of HE (p< 0.001). Since NCCT is so widely used, practically any medical facility may easily ascertain the characteristics required to calculate a score using this grading system. NCCT frequently performed for acute ICH, these findings can be of prognostic value and may assist prioritize anti-expansion therapy to those most likely to benefit. Several limitations were present in the current study. First, the current findings must be confirmed at other healthcare institutions because this study was limited to a single institution and a small sample size. Second, we only included ICH patients who presented within 4 hours after the onset of symptoms; whereas hematoma expansion is known to be less common beyond this time, we do not know if our score would still be relevant for patients who presented after 4 hours.
Conflict of Interests
The authors have no conflict of interest to declare related to this article.
References
- Group GBDNDC. GBD 2015 Neurological Disorders Collaborator Group. Global, regional and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16(11):877-97.
- Steiner T, Salman RA-S, Beer R. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. International J Stroke. 2014;9(7):840-55.
- Hemphill III JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032-60.
- Cai JX, Zhu HC, Yang D. Accuracy of imaging markers on noncontrast computed tomography in predicting intracerebral hemorrhage expansion. Neurol Res. 2020;42(11):973-9
- Hillal A, Ullberg T, Ramgren B, Wassélius J. Computed tomography in acute intracerebral hemorrhage: neuroimaging predictors of hematoma expansion and outcome. Insights Into Imaging. 2022;13(1):180.
- Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(8):1304-5.
- Ng D, Churilov L, Mitchell P. The CT swirl sign is associated with hematoma expansion in intracerebral hemorrhage. AJNR Am J Neuroradiol. 2018;39:232-7.
- Xiong X, Li Q, Yang WS. Comparison of swirl sign and black hole sign in predicting early hematoma growth in patients with spontaneous intracerebral hemorrhage. Med Sci Monit. 2018;24:567-73.
- Selariu E, Zia E, Brizzi M. Swirl sign in intracerebral haemorrhage: definition, prevalence, reliability and prognostic value. BMC Neurol. 2012;12:109.
- Li Q, Zhang G, Xiong X. Black hole sign novel imaging marker that predicts hematoma growth in patients with intracerebral hemorrhage. Stroke. 2016;47:1777-81.
- Li Q, Liu QJ, Yang WS. Island sign an imaging predictor for early hematoma expansion and poor outcome in patients with intracerebral hemorrhage. Stroke. 2017;48:3019-25.
- Li Q, Zhang G, Huang YJ. Blend sign on computed tomography novel and reliable predictor for early hematoma growth in patients with intracerebral hemorrhage. Stroke. 2015;46:2219-23.
- Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, et al. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurol. 2011;76(14):1238-44.
- Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Blas YS, Dzialowski I, et al. Prediction of haematoma growth and outcome in patients with intracerebralhaemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. The Lancet Neurology. 2012;11(4):307-14.
- Kangevari M, Abd-Allah F, Abedi V, Abualhasan A. Global, regional and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet Neurology. 2021;20(10):795-820.
- Li Q, Zhang G, Huang YJ, Dong MX, Lv FJ, Wei X, et al. Blend sign on computed tomography: novel and reliable predictor for early hematoma growth in patients with intracerebral hemorrhage. Stroke. 2015;46:2119-23.
- Li Q, Zhang G, Xiong X, Wang XC, Yang WS, Li KW, et al. Black hole sign: novel imaging marker that predicts hematoma growth in patients with intracerebral hemorrhage. Stroke. 2016;47:1777-81.
- Boulouis G, Morotti A, Brouwers HB, Charidimou A, Jessel MJ, Auriel E, et al. Association between hypodensities detected by computed tomography and hematoma expansion in patients with intracerebral hemorrhage. JAMA Neurol. 2016;73:961-8.
- Romero JM, Brouwers HB, Lu J, Delgado Almandoz JE, Kelly H, Heit J, et al. Prospective validation of the computed tomographic angiography spot sign score for intracerebral hemorrhage. Stroke. 2013;44:3097-102.
- Morotti A, Dowlatshahi D, Boulouis G, Al-Ajlan F, Demchuk AM, Aviv RI, et al. Predicting intracerebral hemorrhage expansion with noncontrast computed tomography: the BAT score. Stroke. 2018;49(5):1163-9.
- Huang Y, Zhang Q, Yang M. A reliable grading system for prediction of hematoma expansion in intracerebral hemorrhage in the basal ganglia. BioScience Trends. 2018;12(2):193-200.
- Chen S, Zhao B, Wang W, Shi L, Reis C, Zhang J. Predictors of hematoma expansion predictors after intracerebral hemorrhage. Oncotarget. 2017;8(51):89348.
- Broderick JP, Diringer MN, Hill MD, Brun NC, Mayer SA, Steiner T, et al. Determinants of intracerebral hemorrhage growth: an exploratory analysis. Stroke. 2007;38(3):1072-5.
- Dowlatshahi D, Smith EE, Flaherty ML, Ali M, Lyden P, Demchuk AM, et al. Small intracerebral haemorrhages are associated with less haematoma expansion and better outcomes. International J Stroke. 2011;6(3):201-6.
- Li Q, Huang YJ, Zhang G, Lv FJ, Wei X, Dong MX, et al. Intraventricular hemorrhage and early hematoma expansion in patients with intracerebral hemorrhage. Scientific Reports. 2015;5(1):11357.
- Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. InBrain Edema XIII. 2006;65-8.
- Steiner T, Diringer MN, Schneider D, Mayer SA, Begtrup K, Broderick J, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact and effect of hemostatic therapy with recombinant activated factor VII. Neurosurg. 2006;59(4):767-74.
- Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Tanaka R. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke. 1998;29(6):1160-6.
- Silva Y, Leira R, Tejada J, Lainez JM, Castillo J, Dávalos A. Stroke Project, Cerebrovascular Diseases Group of the Spanish Neurological Society. Molecular signatures of vascular injury are associated with early growth of intracerebral hemorrhage. Stroke. 2005;36(1):86-91.
- Cai J, Zhu H, Yang D, Yang R, Zhao X, Zhou J, et al. Accuracy of imaging markers on noncontrast computed tomography in predicting intracerebral hemorrhage expansion. Neurological Res. 2020;42(11):973-9.
- Brouwers HB, Chang Y, Falcone GJ, Cai X, Ayres AM, Battey TW, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurology. 2014;71(2):158-64.
- Wang X, Arima H, Al-Shahi Salman R, Woodward M, Heeley E, Stapf C, et al. Clinical prediction algorithm (BRAIN) to determine risk of hematoma growth in acute intracerebral hemorrhage. Stroke. 2015;46(2):376-81.
Article Type
Research Article
Publication History
Received Date: 09-10-2024
Accepted Date: 29-10-2024
Published Date: 07-11-2024
Copyright© 2024 by Fatima SA, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Fatima SA, et al. A Five-Point Score Grading System for Predicting Early Hematoma Expansion in Patients with Spontaneous Intracerebral Hemorrhage. J Neuro Onco Res. 2024;4(3):1-10.

Figure 1: Flowchart.

Figure 2: Calculating volume of hematoma in NCCT scan brain in intracranial bleed using ABC/2=volume in ml. A = maximum length in cm. B= maximum width in cm perpendicular to the line of length in the same CT slice. C= The longest cranio-caudal measurement on the coronal section as C.

Figure 3: A: A 59-year-old man presented with a sudden onset of right-sided weakness. On Admission Axial Noncontrast Computed Tomography (NCCT) image performed 1.5 hours after hospital admission showed a left basal ganglia bleed with a swirl sign; B: Follow-up Axial Non-Contrast Computed Tomography (NCCT) 8 hours later showed a significant hematoma expansion.

Figure 4: A: 68-year-old woman presented with a sudden onset of aphasia, right-sided weakness. On admission a axial noncontrast computed tomography (NCCT) image performed 3 hours after hospital admission showed left basal hematoma with a black hole sign; B: Follow-up axial non-contrast computed tomography (NCCT) 7 hours later showed a significant hematoma expansion with intra-ventricular extension.

Figure 5: A: 66-year-old man presented with a sudden onset of left-sided weakness. On admission axial non-contrast computed tomography image performed 2 hours after hospital admission showed a lobar hematoma with an island sign (arrows); B: Follow-up CT 6 hours later, it had significant hematoma expansion.
Gender | Number (n) | Percentage (%) |
Male | 60 | 62.5 |
Female | 36 | 37.5 |
Total | 96 | 100 |
Male:Female=1.7:1 | ||
Comorbidity |
|
|
Hypertension | 75 | 78.1 |
Diabetes | 37 | 38.5 |
Chronic kidney disease | 33 | 35.1 |
Smoking | 23 | 24 |
Dyslipidemia | 19 | 19.8 |
Alcohol intake | 3 | 3.1 |
Parameter | ||
Hematoma location | Capsuloganglionic | 50 | 52.1 |
Lobar | 35 | 36.5 | |
Infratentorial | 11 | 11.5 | |
IVH | Present | 54 | 56.3 |
Absent | 42 | 43.8 | |
SAH | Present | 3 | 3.1 |
Absent | 93 | 96.9 | |
Baseline hematoma volume | < 30 ml | 57 | 59.4 |
≥ 30 ml | 39 | 40.6 | |
Midline shift | Present | 74 | 77.1 |
Absent | 22 | 22.9 | |
Island sign | Present | 31 | 32.3 |
Absent | 65 | 67.7 | |
Swirl sign | Present | 22 | 22.9 |
Absent | 74 | 77.1 | |
Black hole sign | Present | 29 | 30.2 |
Absent | 67 | 69.8 |
Table 1: The baseline demographics, clinical, radiological characteristics.
Variable | HE | No HE | P-value |
Baseline hematoma volume (ml); Mean±SD | 44.1±26.34 | 12.2±16.32 | <0.001* |
IVH, n (%) | 41 (77.4) | 13 (30.2) | <0.001* |
SAH, n (%) | 2 (3.8) | 1 (2.3) | 0.685 |
Island sign, n (%) | 28 (52.8) | 3 (7.0) | <0.001* |
Swirl sign, n (%) | 16 (30.2) | 6 (14.0) | 0.049* |
Black hole sign, n (%) | 21 (39.6) | 8 (18.6) | 0.026* |
Table 2: Baseline demographic and NCCT imaging characteristics of study population with and without hematoma expansion.
Variable | IS/SS/BS (+) | IS/SS/BS (-) | P-value |
Imaging characteristics as per Island Sign | |||
Baseline hematoma volume (ml); Mean±SD | 40.9±27.03 | 24.5±26.08 | 0.005* |
HE, n (%) | 28 (90.3) | 25 (38.5) | <0.001* |
Imaging characteristics as per Swirl Sign | |||
Baseline hematoma volume (ml); Mean±SD | 37.9±21.62 | 25.4±23.97 | 0.031* |
HE, n (%) | 16 (72.7) | 37 (50.0) | 0.049* |
Imaging characteristics as per Black hole Sign | |||
Baseline hematoma volume(ml); Mean±SD | 35.8±21.98 | 26.1±18.85 | 0.045* |
HE, n (%) | 21 (72.4) | 32 (47.8) | 0.026* |
IS: Island Sign; Swirl Sign, Black hole Sign *Statistically significant Difference (P-value<0.05) | |||
Table 3: Imaging characteristics as per Island sign, swirl sign, black hole sign.
Parameter | OR | 95% CI | P-value |
Baseline hematoma volume ≥ 30 ml | 1.9 | 1.41-2.74 | 0.039* |
IVH | 4.5 | 2.79-6.82 | 0.008* |
Island sign | 13.7 | 10.15-16.37 | <0.001* |
Swirl sign | 5.2 | 3.72-6.53 | <0.001* |
Blackhole sign | 9.4 | 7.4-11.62 | <0.001* |
OR: Odds Ratio; CI: Confidence Interval; *Statistically Significant (P value<0.05) | |||
Table 4: Multivariate logistic regression depicting predictors for hematoma expansion.
Component | Points |
Baseline hematoma volume (ml) | |
≥ 30 | 1 |
< 30 | 0 |
IVH | |
Present | 1 |
Absent | 0 |
Island sign | |
Present | 1 |
Absent | 0 |
IVH | |
Present | 1 |
Absent | 0 |
Table 5: Summary of the HE prediction grading system.
Total score | No. of Patients | HE | Risk of HE (%) |
0 | 27 | 2 | 7.4 |
1 | 16 | 6 | 37.5 |
2 | 20 | 15 | 75.0 |
3 | 14 | 12 | 85.7 |
4 | 15 | 14 | 93.3 |
5 | 4 | 4 | 100 |
Table 6: Risk of Hematoma Expansion (HE) according to the prediction grading system.


