Alteration of Endocrine Functions and Reproductive Outcome Following Consumption of Edible Crops Grown Around E-Waste Dumpsite Using Albino Wistar Rat Model
Johnson JT1*, Okon EA2
1Department of Biochemistry, Faculty of Science, Federal University Otuoke, Bayelsa State, Nigeria
2Department of Chemical Science, Faculty of Applied Science, Akwa Ibom State Polytechnic, Ikot Osurua, Akwa Ibom State, Nigeria
*Correspondence author: Johnson JT, Department of Biochemistry, Faculty of Science, Federal University Otuoke, Bayelsa State, Nigeria; Email: johnsonjt@fuotuoke.edu.ng
Published Date: 09-06-2023
Copyright© 2023 by Johnson JT, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
| Received 12 May, 2023 | Accepted 02 Jun, 2023 | Published 09 Jun, 2023 |
Abstract
Electronic waste (e-waste) is the term used to describe old, end-of-life or discarded appliances using electricity in one form or the other which are destined for reuse, resale, salvage, recycling but most importantly disposal. It contains plethora of metals as well as many other toxic chemicals. Research has confirmed endocrine disruption potentials of e-waste resulting from occupational and environmental exposures. However, not much is known about the effects or the hazard of consuming crops from e-waste dumpsite on health (reproductive health/outcome). Hence, this study aimed at evaluating alterations in pituitary-gonadal hormones levels, histology of the gonads and sperm parameters associated with consumption of food crops harvested around e-waste dumpsite. The area selected for the study was Yenagoa, the Bayelsa State capital which is home to over 350,000 thousand people with hundreds of electronic equipment’s retail stores. Samples; soil, plant (maize, fruited pumpkins, scent leaf), water and sea foods were collected around e-waste dumpsites for laboratory screening and after which the edible plants and some sea foods were obtained and prepared into feed which was used to feed the laboratory experimental animals (albino wistar rats) for a period of three months ad libitum. A standard animal feed was formulated from the powder of the above-mentioned materials and the feed formulated from plants from farm around E-waste dumpsite was used to feed the animals in group B (Test group). Group A serve as control and were fed with commercial pellet. The formulated feeds contained maize as its main source of energy. The samples were screened for the presence of toxic metals such as; lead, mercury, cadmium, nickel, copper, chromium, using a wavelength Perkin Elmer 1100 Atomic Absorption Spectrometer (Oxford Instrument, X-MET8000 series). Twenty-eight rats (fourteen each of male and female) of albino wistar strain, weighing 50-100 g at the beginning of the experimental period were used for this study divided into two groups of fourteen animals each per group (7 male and 7 female) with a wire mesh separating the sexes which was later removed in order to allow animals in the test group to meet for procreation two months to end of the experimental period. Male and female fertility hormones; Luteinizing Hormone (LH), Follicle Stimulating Hormone (FSH), Testosterone (TESTO) as well as Progesterone (PROG), Estrogen (EST) and Thyroid Stimulating Hormone (TSH) were determined using Enzyme Linked Immunosorbent Assay (ELISA) methods. Sperm analysis was also done for the male animals alongside histology of the testes. Serum levels of Testosterone, Luteinizing Hormone and Follicle Stimulating Hormone for males, Estrogen for females in the test group (exposed group) were significantly (p<0.05) lower compared with the control group. In contrast, serum TSH were significantly (p<0.05) higher in e-waste exposed population (both male and female) compared with the control group. More so, chromium, Cadmium, Nickel, Lead concentration in our harvested crop used correlated significantly but negatively with testosterone, LH, FSH, TSH and estrogen fluctuations as well as alterations in sperm parametres with 86% of non-motile sperm cells in exposed group. This effect was elucidated also by alteration in the histology of the testes.
Conclusively, this study showed a strong correlation between consumption of food crops cultivated around e-wastes dumpsites and disruption of fertility hormones, poor sperm outcome (male), altered gonadal histology as well as impaired reproduction expectation in this research.
Keywords: Endocrine Disruptors; E-Wastes; Toxic Metals; Reproductive Hormones; Reproductive Outcome
Introduction
Electronic Waste or e-waste is the term used to describe old, end-of-life or discarded appliances using electricity in one form or the other which are destined for reuse, resale, salvage, recycling but most importantly disposal. It includes computers, cell phones (cell phone batteries), printers, fax machines, scanners, MP3/CD players, cameras, washing machines, Televisions (TVs), consumer electronics, fridges, medical equipment, etc., which have been disposed of by their original users or the repairers. Electronic Waste or “E-waste” is used as a generic term embracing all types of waste containing electrically powered components. It contains both valuable materials as well as hazardous materials which require special handling, recycling and disposal methods.
In the 1990s, many governments around the globe set up e-waste ‘recycling’ systems but many countries did not have the capacity to deal with the sheer quantity of e-waste they generated or with its hazardous nature. Thus, they began exporting the problem to developing countries where laws to protect workers and the environment are inadequate or not enforced. It is also cheaper to ‘recycle’ waste in developing countries than in the developed world; the cost of glass-to-glass recycling of computer monitors in the US is ten times more than in China and fifty times more in Ghana.
Demand in Asia for electronic waste began to grow when scrap yards found they could extract valuable substances such as copper, iron, silicon, nickel and gold. Electronic waste became routinely exported by developed countries to developing ones, often in violation of international laws. Inspections of 18 European seaports in 2005 found that as much as 47 percent of waste destined for export, including e-waste, where illegal. In the UK alone, at least 23,000 metric tons of undeclared or ‘grey’ market electronic waste was shipped in 2003 to the Far East; India, China and even Africa (Nigeria inclusive). In the US, it is estimated that 50-80 percent of the waste collected for recycling is being exported in this way and this practice is legal because the US has not ratified the Basel Convention. However, research show that the laws are not enforced, but for a developing country like Nigeria where such laws may not even be in place, e-waste still arriving in our ports are huge. Hence, the increasing ‘market penetration’ in the developing countries, ‘replacement market’ in the developed countries and ‘high obsolescence rate’ make e-waste one of the fastest waste streams in developing countries including ours [1]. This new kind of waste is posing a serious challenge in disposal and recycling to both developed and developing countries. While having some of the world’s most advanced high-tech software and hardware developing facilities, India’s recycling sector can be called medieval [2]. The dumping of e-waste, particularly computer waste, into India, China, Pakistan, Ghana and Nigeria from developed countries (‘green passport’ according to Gutierrez because the latter find it convenient and economical to export waste, has further complicated the problems with waste management especially in these countries with little or no technical knowhow on how to manage these wastes and its outcomes [1,3]. All these have made e-waste management an issue of environmental and a public health concern especially in a developing country like Nigeria.
The main cause or rather reason for the increasing e-waste is the increased number of electronic products as well as e-waste includes; development, technology, human mentality and consumer purchasing power, and; quality and population growth. Sequel to the above, the present investigation is aimed at scientifically establishing a body of scientific data on the leaching of hazardous chemical from e-waste, effect of e-waste (leached chemicals) on our environment hitherto the possible associated potential reproductive health hazard on terrestrial life including man using Yenagoa (Bayelsa State) as the study area.
Electronic waste is made of a multitude of components with some containing toxic substances that have an adverse impact on human health and the environment if not handled properly. Often, these hazards arise due to the improper recycling and disposal processes used and E-waste e.g., computer, television ray tube and some hospital equipment contain highly toxic chemicals like lead, cadmium, mercury, beryllium, BFR, polyvinyl chloride and phosphor compounds [4,5]. The health associated risks with lack of proper handling of e-waste includes; toxic effects on various systems in the body such as the central (organic affective syndrome) and peripheral nervous systems (motor neuropathy), the reproductive systems (male and female) [6]. United Nations Committee on environment has this to say; “E-waste is a toxic legacy of our digital age; our electronics waste is polluting soil, drinking water, food crops and harming ecosystems around the world. It’s time to know the extent of harm and damage of e-waste in our respective community and health (reproductive health)”. Thus, since such body of scientific information (scientific data on adverse reproductive health effects of e-waste) is inadequate in Nigeria, there is need to trace the distribution pattern of the hazardous chemicals from this waste into our food chain and its possible effects on terrestrial lives (human’s health).
This raises concerns about the immediate and generational dangers to the environment and humans and thus; justifies this study since it has scientifically established a body of scientific data on the leaching pattern of these toxic chemical from e-waste, its potential effect on reproductive health and other health risks even to the unborn generations.
Methodology
Study Area
The area selected for the study was Yenagoa, the Bayelsa State capital which is home to over 350,000 thousand people with hundreds of electronic equipment retail stores (Fig. 1).
Figure 1: Map of Yenagoa, Bayelsa state (Study Area) [7].
Collection and Analysis of Samples
Questionnaires designed on a four-linked scale and standardized. The questionnaires were randomly distributed to dealer and repairer of one or more form of electrical appliances stating how and where they dispose of end-of-life electrical appliances, scavengers were also interviewed. The outcome from the questionnaires determined the specific sites in the above mentioned LGA where samples (soil, plant, water and sea foods) were collected (Azikoro village and Imiringi Road) for laboratory screening and after which the edible plants and some sea foods were obtained and prepared into feed which was used to feed the laboratory experimental animals for a period of three months.
Sample Preparation
The plant samples (maize, fruited pumpkin, scent leaf) were washed with running tap water and rinsed with distilled water to remove sand and other possible contaminants. The leaves were cut into smaller piece with stainless steel kitchen knife and allowed to dry at room temperature (25℃) alongside maize grains. Similar preparation process was applied to fish obtained from a nearby pond which was used as part of this studies, the fish was oven dried at 45℃ until a constant weight was obtained. The dried samples were pulverized, using a laboratory blender followed by sieving through a 0.5 mm mesh size sieve to obtain a fine powder but uniform particle size. Each plant sample was labeled and stored in polyethylene bags and stored at ambient temperature until when they were required for various analyses and feed formulation. The soil samples were also stored in well labeled polyethylene bags until required for analysis. The sample digestion method by Francek, et al., was adopted for the extraction of trace heavy metals in the study [8].
Experimental Feed Formulation
A standard animal feed was formulated from the powder of the above-mentioned materials. They were measured, mixed properly to obtain a homogenous mixture then constituted into a feed and the feed formulated from plants from farm around E-waste dumpsite was used to feed the animals in group B (Test group). The group A serves as control and was fed with commercial rat pellet. The formulated feeds contained maize as its main source of energy. Feed formulation was done using the method of Johnson, et al., [9].
Chemical Characterization of the e-waste
The samples were screened for the presence of toxic metals such as; lead, mercury, Cadmium, nickel, copper, chromium, using a wavelength Perkin Elmer 1100 Atomic Absorption Spectrometer (Oxford Instrument, X-MET8000 series). Samples were oven-dried at 80℃ for 18-20 hours. The soil, plants, water and fish samples were screened for the presence of heavy metals such as; Lead, Chromium, Cadmium, Nickel, Coppers and Cobalt. Other toxic compounds assay for including; brominated flame retardant and dioxins were also assayed for using various standard laboratory methods as approved by the Association of Official Analytical Chemists (AOAC, 2013) [10]. Sample preparation and heavy metal analysis were conducted based on the standard method by American Public Health Association [11].
Experimental Animals
Twenty-eight rats (fourteen each of male and female) of albino wistar strain, weighing 50-100 g at the beginning of the experimental period were used for this study. The rats were obtained from the Department of Biochemistry, Faculty of Science, Niger Delta University, Amasoma, Bayelsa State Nigeria. The animals were allowed to acclimatize for a period of one week in the Department of Biochemistry, Federal University Otuoke, Bayelsa State, animal house after which they were reweighed and housed in plastic cages with a wire-mesh bottom and top (North Kent Co. Ltd), under controlled environmental conditions of temperature (28±2℃), relative humidity (50±5%) and 12-hour light/dark cycle and adequately ventilated.
Animal Grouping and Experimental Protocol
The study comprised twenty-eight animals divided into two groups of fourteen animals each per group (male and female) with a wire mesh separating the sexes which was removed in the course of the study in order to allow animals in the test group to meet for procreation, two months to end of the experimental period. The normal control group was maintained on with commercial chow or pellet while the other group was placed on the feed formulated in the laboratory from plants, water and sea food (fishes) obtained around e-waste dumpsites, feed and water were provided ad libitum. The feeding schedule spanned to three months as shown in Table 1 below.
Group | Type of Feed | Number of Animals | Duration |
Normal Control (Male and female) | Prepared feed | 14 | Three Months |
TEST group I (Male and female) | Prepared feed | 14 | Three Months |
Table 1: Experimental design.
Collection of Blood and Tissue Samples for Analysis
The animals were sacrificed twelve hours after the last day of the studies; whole blood was collected from the heart via cardiac puncture using sterile syringes and needles in accordance with guidelines of the European Convention for the protection of vertebrate animals and other scientific purposes ETS-123 [12]. The blood samples were put into plain sample tubes. Serum was obtained from the clotted samples by letting it stands for 2 hours at room temperature to clot prior to centrifugation at 4000 rpm for 10 minutes using MSE England bench top centrifuge.
Sera obtained from each sample was gently separated using Pasteur pipettes and dispensed into respective dry specimen bottles labeled accordingly and were kept frozen in a freezer until needed for the various biochemical assays. Semen from the male was obtained for semen analyses while the testes were excised from the animals into sterile universal bottles with formaldehyde for histomorphological assessment.
Biochemical Evaluation
All Biochemical investigations were done using standard methods with the aid of Randox kits and an AJ-Semi-Auto Biochemical Analyzer. Hormonal assay was done using ELISA alongside immunological evaluation, the volume, percentage of viability and mobility was also measured on the semen alongside its morphology.
Histopathological Examination
The principle presented by Drury and Wallington was used to examine the histology of testes of rats in the control and test groups [13]. Tissues fixed in 10% buffered formaldehyde were dehydrated via increasing concentration of ethanol (70, 90 and 95%). FULGEN’s staining technique was also adopted to specifically reveal some special features and thus pathologies in the testes like DNA etc. High powered photographs of the sections of the testes were taken in bright field at X100 and X400.
Statistical Analyses
Data obtained was expressed as Mean ± SEM and analysis was done using the Analysis of Variance ‘ANOVA; f-ratio’ and Statistical Package for Social Scientists (SPSS version 21.0), graph pad etc. Values at P<0.05 were considered significant in comparison with appropriate controls [14].
Results
Result of Male Hormonal Profile
The results of the effect of feed formulated from edible crops obtained around E-waste dumpsite on male hormonal profile are as presented on Table 2.
Testosterone
The serum concentration of testosterone of animals in the test group (1.01 ± 0.12) was significantly (p<0.05) lower than that of the control group (7.50 ± 1.71).
Follicle Stimulating Hormone
The serum concentration of testosterone of animals in the test group (0.11 ± 0.01) was significantly (p<0.05) lower than that of the control group (0.56 ± 0.11).
Luteinizing Hormone
The serum concentration of LH of animals in the test group (0.09 ± 0.00) was significantly (p<0.05) lower than that of the control group (0.86 ± 0.14).
Thyroid Stimulating Hormone
The serum concentration of TSH of animals in the test group (0.84 ± 0.00) was significantly (p<0.05) higher than those of the control group (0.009 ± 0.00).
Result of Female Hormonal Profile
The results of the effect of feed formulated from edible crops obtained around E-waste dumpsite on female hormonal profile are as presented on Table 3.
Estrogen (E2)
The serum concentration of E2 of animals in the test group (30.36 ± 0.20) was significantly (p<0.05) lower than that of the control group (46.455 ± 0.24).
Progesterone
The serum concentration of progesterone of animals in the test group (21.280 ± 0.11) showed no significant (p>0.05) changes compared with that of the control group (18.268 ± 0.10).
Luteinizing Hormone (LH)
The serum concentration of LH of animals in the test group (2.264 ± 0.04) recorded no significant (p>0.05) different compared with the control group (2.967 ± 0.01).
Follicle Stimulating Hormone (FSH)
The serum concentration of FSH of animals in the test group (0.412 ± 0.01) showed no significant (p>0.05) changes compared with the control group (0.423 ± 0.01).
Thyroid Stimulating Hormone (TSH)
The serum concentration of TSH of animals in the test group (0.062 ± 0.00) was significantly (p<0.05) higher when compared with the control group (0.004 ± 0.00).
Group | Testosterone (µmol/L) | FSH (µmol/ml) | LH (µm/ml) | TSH |
A | 7.5 ± 1.71 | 0.56 ± 0.11 | 0.86 ± 0.14 | 0.009 ± 0.00 |
B | 1.01 ± 0.12* | 0.11 ± 0.01* | 0.09 ± 0.00* | 0.84 ± 0.00* |
Table 2: Effect of feed formulated from plants obtained around E-waste dumpsite on male’s hormonal profile. Values are expressed as Mean±Standard Error of Mean (SEM); n=7; Key: A: Control; B: Test Group; FSH: Follicle Stimulating Hormone; LH: Luteinizing Hormone; TSH: Thyroid Stimulating Hormone; *: Significant at P<0.05 compared with the control group.
Group | E2 (µmol/ml) | Prog (ng/ml) | LH (µ/ml) | FSH (µ/ml) | TSH |
A | 46.455 ± 0.24 | 18.268 ± 0.10 | 2.967 ± 0.01 | 0.423 ± 0.01 | 0.004 ± 0.00 |
B | 30.36 ± 0.20 | 21.280 ± 0.11 | 2.264 ± 0.04 | 0.412 ± 0.01 | 0.062 ± 0.00* |
Table 3: Effect of feed formulated from plants obtained around E-waste dumpsite on female’s hormonal profile. Values are expressed as Mean±Standard Error of Mean (SEM); n=7; Key: A: Control; B: Test Group; E2: Estrogen2; Prog: Progesterone; FSH: Follicle Stimulating Hormone; LH: Luteinizing Hormone; TSH: Thyroid Stimulating Hormone; *: Significant at P<0.05 compared with the control group.
Sperm Analysis
Analytical sperm tests parametres revealed that the test group had a significantly lower sperm count (90,000) than the control group (271,000). Normal spermatozoa of the test group were 95% of the total leaving 5% as abnormal. The control group had 97% of normal cells and 3% abnormal sperm cells. The semen volume of the test group (0.4 ml) was slightly lesser than that of the control group (0.5 ml). 86% of sperm cells in the test group were non-motile while only 5% of the control was non-motile. All other parameters were same for both groups (Table 4).
Group | Volume | PH | Colour | Turbidity | Active | Sluggish | Non-Motile | Normal Cells | Abnormal Cells | Sperm Count |
A | 0.5 | 7.7 | Greyish White | Turbid | 90 | 5% | 5% | 97% | 3% | 271,000 |
B | 0.4ml | 7.5 | Greyish White | Turbid | 5% | 9% | 86% | 95% | 5% | 90,000 |
A-Control; B-Test; Time Produced: 9:30am; Time Received: 10:20am; Time Examined: 10:50am | ||||||||||
Table 4: Effect of feed formulated from plants obtained around E-waste dumpsite on sperm’s parameters.
Discussion
This study evaluated the effects of e-waste contaminated environment on the food chain and hence, the reproductive health trait its posse to man. Albino wistar rats’ model was used; where food crops grown around the e-waste sites were administered to the labouratory rats. Hormonal profiles, semen analysis and histological assessment of tissues were carried out and their levels or outcomes compared with a control group in order to ascertain the above stated effects. The results of our experiments revealed a significant increase in Thyroid stimulating hormone in the male and female test groups compared to the control group. This effect of e-waste pollution on the test groups was similarly observed in another study by Eguchi, et al. [15]. The observed significantly lower levels of testosterone, estrogen, luteinizing hormone and follicle stimulating hormone in e-waste exposed animals compared with the unexposed animals may be associated with the endocrine disrupting effects of known e-waste-borne chemicals/metals and it resulted in lack of procreation after the first generation (evidence by absence of litres or offspring). Electronic waste (e-waste) exposure incurs a risk of endocrine disruption to affected organisms as e-waste is known to contain thousands of toxic chemicals and metals capable of negatively affecting associated endocrine hormones even at low plasma concentrations [16-18]. Studies have shown that toxic metals exposure causes plethora of pathological conditions to living organisms it may (heavy metals) caused detrimental effects to different body organs and systems of humans and hence, showed that heavy metals may affect the male reproductive system directly by targeting specific reproductive organs, or the process of spermatogenesis or can also affect the male reproductive system indirectly by acting on the neuroendocrine system [19-21]. Heavy metals are also known endocrine disruptors (example, arsenic, lead, boron, mercury, cadmium, antimony, aluminum, cobalt, chromium and lithium) have been found to exert adverse effects on the reproductive axis of human and experimental animals. Men working in battery plants and exposed to toxic levels of lead demonstrated adverse effects on their reproductive capacity [22]. The endocrine disrupting properties of heavy metals may be the sole cause of the differences in the hormonal concentrations between the animals administered with e-waste contaminated food crops (test group) and the animals in the control group. Disruption of Sertoli cells during fetal development can make these effects long-lasting. The number of Sertoli cells determines the number of sperms produced in adulthood, since each Sertoli cell can only support a finite number of germ cells which would later develop into sperm. The fetal, neonatal and pre-pubertal period are the periods when Sertoli cells proliferate and these periods are particularly sensitive to the adverse effects of heavy metals [22]. Though the testicular histology showed no visible anatomical damage may be due to short duration of exposure (Fig. 2), the effect of e-waste components on Sertoli cells may be responsible for the massively lower sperm count of the test group (as seen on Table 5) in comparison with the control group as recorded in our study and subsequent absents of litres from the first generation.
Sample/Metal | Chromium (ppm) | Cadmium (ppm) | Nickel (ppm) | Copper (ppm) | Lead (ppm) | Cobalt (ppm) |
Maize | 0.0342±0.00 | 0.0456±0.00 | 0.8395±0.00 | 0.0924±0.00 | 0.0585±0.00 | 0.1226±0.00 |
Scent Leaf | 0.0354±0.00 | 0.7942±0.00 | 3.8778±0.00 | 0.1147±0.00 | 0.0375±0.00 | 0.1149±0.00 |
Fluted Pumpkin | 0.0333±0.00 | 0.7715±0.00 | 0.9272±0.00 | 0.0849±0.00 | 0.0588±0.00 | 0.1478±0.00 |
Soil | 0.0267±0.00 | 0.5486±0.00 | 5.0367±0.00 | 0.0461±0.00 | 0.0471±0.00 | 0.1515±0.00 |
WHO | 0.05 | 0.003 | 0.07 | 2.0 | 0.01 | 0.002 |
Table 5: Results of heavy metals analysis for E-waste site samples (sampling location). Values are expressed as Mean±Standard Error Mean (SEM).
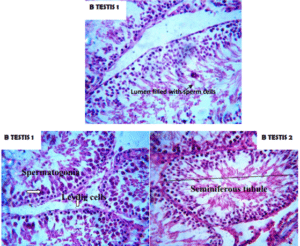
Figure 2: Transverse section of the testis stained with haematoxylin and eosin × 400 magnification, slides show normal somniferous tubules with capsule lined by mature spermatogonia type A and B. Also seen is a company of Leydig cells within the interstitium. Conclusion: Features consistent with normal histology.
Conclusion
Results obtained after labouratory assay and statistical analysis of samples from the group exposed to e-waste contaminated feed and the control group (not exposed), infers that e-waste exposure begets negative consequences on various reproductive indices which are used as reproductive markers. Many of the various indicators assayed for, exhibited significantly (p<0.05) different values from the control group which points to a shift from normal in the e-waste exposed animals. Conclusively, the study showed a strong correlation between consumption of food crops cultivated around e-wastes dumpsites and disruption of fertility hormones, poor sperm outcome (male) as well as impaired reproduction expectation in this research.
Limitations of the Study
The following among other limitations were encountered in the course of this studies;
- The electronic wastes where not classified: All forms were dump together
- Some of the sites (the dump sites and farm lands) where difficult to accessed
- Most of the scrap scavengers and some of the electronic repairers where not cooperating in the area of information sharing
Acknowledgment
I acknowledge and appreciate Tertiary Education Trust Fund (TETFund) for funding this research work in full under “2021 TETFund Institution-Based Research (IBR) Intervention”.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- Wankhede KK. Britain’s environment agency confirmation of huge e-waste outflow to India. Toxics Link. 2005;26(5):15-7.
- Swerts T. Waste; opportunity or a hazard? – The importance of a progressive Indian e-waste policy. Toxics Link. 2006;27(1):26-31.
- Harder B. Toxic “E-waste” gets cashed in poor nations. The hazard wave. 2005;3(6):7.
- Beary H. Bangalore faces e-waste hazards. The hazard wave. 2008;3(6):15-7.
- Sinha S. Downside of the digital revolution. Toxics Link. 2007;28(12):37-45.
- Harrington JM, Baker EL. Occupational and environmental health and society. In: David AW, Timothy MC, John DF, Edward JB, Editors. Oxford Textbook of Medicine. Oxford University Press. 2003;4(1):956-60.
- Google Map Data. 2017. [Last accessed on May 31, 2023]
https://www.mapsstreetview.com/Nigeria/BayelsaState/roadmap.php
- Francek MA, Makimaa B, Pan V, Hanko JH. Small town lead levels: A case study from the homes of pre-schoolers in Mt. Pleasant, Michigan. Environmental Pollution. 1994;84(2):159-66.
- Johnson JT, Chibuike PO, Ifeakor OD. Formulation and nutrient assessment of poultry feed from domestic waste and its effect on the growth of poultry birds. Int J Agriculture Technol. 2022;2(1):1-7.
- Official methods of Analysis. 12th Edition, Association of Official Analytical Chemists, Washington DC. 2013;23-54.
- American Public Health Association (APHA). American Water Works Association and Water Environment Federation. Standard methods for the examination of water and wastewater, 20th Washington, DC. 1999;94-111.
- European Treaty. European convention for the protection of vertebrate animals uses for experimental and other scientific purposes. Strasbourg EST-123. 2005.
- Drury RA, Wallington EA. Carleton’s histological technique 5th ed. New York: Churchill Livingstone. 1980.
- Welkowitz W, Deutsch S, Akay M. Biomedical instruments: theory and design. 2nd Washington: Howard Press. 2006;67-71.
- Eguchi A, Nomiyama K, Tue NM, Trang PT, Viet PH, Takahashi S, et al. Residue profiles of organohalogen compounds in human serum from e-waste recycling sites in North Vietnam: association with thyroid hormone levels. Environmental Res. 2015;137:440-9.
- Cobbing M. Toxic tech: not in our backyard. Uncovering the hidden flows of e-waste. Amsterdam: Greenpeace International. 2008;3(1)56-63.
- Sthiannopkao S, Wong MH. Handling e-waste in developed and developing countries: initiatives, practices and consequences. Sci Total Environ. 2013;463:1147-53.
- United Nations Environment Programme (UNEP). Basel convention on the control of transboundary movements of hazardous waste and their disposal. Châtelaine, Switzerland: United Nations Environment Programme. 2014.
- Terada C. Recycling electronic wastes in Nigeria: putting environmental and human rights at risk. Northwestern J Intl Human Rights. 2012:10:154-72.
- Barabanic D, Rupnik MC, Klemencic AKD. Negative impact of endocrine disrupting compounds on human reproductive health. Reprod Fertil Develop. 2011;23:413-6.
- Pizent A, Tariba B, Zivkovic T. Reproductive toxicity of metals in men. Arh Hig Rada Toksikol. 2012;63:35-46.
- Adaramodu AA, Osuntogun AO, Ehi-Eromosele CO. Heavy metal concentration of surface dust present in e-waste components: the Westminster electronic market, Lagos case study. Res Environ. 2012;2:9-13.
This work is licensed under a Creative Commons Attribution 2.0 International License.


