Dental Pulp Therapies: Indirect and Direct Capping and Pulp Regeneration
Michel Goldberg1*
1Department of Oral Biology, Faculty of Fundamental and Biomedical Sciences, INSERM UMR-S 1124 Paris Cite University, France
*Corresponding Author: Michel Goldberg, Department of Oral Biology, Faculty of Fundamental and Biomedical Sciences, INSERM UMR-S 1124 Paris Cite University, France; Email: mgoldod@gmail.com
Citation: Goldberg M. Dental Pulp Therapies: Indirect and Direct Capping and Pulp Regeneration. Jour Clin Med Res. 2022;3(1):1-11.
Copyright© 2022 by Goldberg M. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
| Received 05 Dec, 2021 | Accepted 31 Jan, 2022 | Published 07 Feb, 2022 |
Abstract
Specific dental therapies refer to pulp healing and regeneration. These therapies are related to
- Indirect and direct pulp capping
- Vital amputation (conventional endodontics)
- Treatment of infected non-vital teeth and pulp regeneration
In the two first cases, cells differentiate into functional odontoblasts producing reactionary dentin. The third option leads to the formation of tertiary (reparative) dentin. The healthy pulp includes a series of cells: fibroblasts, stem cells, dendritic cells, histiocytes /macrophages, B and T-lymphocytes, vascular cells involving capillaries, arterioles and veins, lymph vascularization and pulp innervation. Direct or indirect pulp capping, promote a tubular reactionary dentin, beneath a calciotraumatic line. If the pulp is partially or totally infected, after disinfection and/or cleaning solutions, residual apical cells, recolonize the lumen, leading to the activation of pericytes and apposition of parietal dentin. Apexogenesis (root lenghtening) and apexification (apical closure) contribute to dental therapies (re-formation and/or pulp regeneration). The purpose of this review is to summarize the beneficial effects of indirect and direct capping and their usefulness for pulp regeneration.
Keywords
Pulp Cells; Fibroblasts; Stem Cells; Dendritic Cells; Histiocyte/Macrophage; Capillaries; Pericytes; Apexogenesis; Apexification
Introduction
Dental pulp is a non-mineralized tissue implicated in formative, nutritive and reparative activities. Dental pulp therapies are classified into three categories; 1-direct or indirect pulp capping (non-infected pulp), 2-vital pulp amputation (endodontic therapies) and 3-treatment for non-vital teeth aiming to regenerate the dental pulp. Distinct differences underline the crown from the root.
Coronal pulp: is the part of pulp located in the crown. There are six surfaces in the coronal pulp: occlusal or roof pulp, floor, mesial, distal, buccal and lingual, or palatal. Dentin formation is due to odontoblasts, responsible for the activation of calcification process, leading to closure of the pulp exposure under a dentinal bridge. These bridges are crossed by tunnels and display labelling for osteopontin. This formation as a reaction to caries, fractures and noxious events.
Root pulp: is related to the pulp that remains in the root(s). Anterior and posterior teeth have single or multiple branches of pulp in the pulp canals. The radicular pulp is not straight but vary in size, shape and number. They are connected with apical foramen accessory canals. During root formation the apical opening is wide open, limited by an epithelial diaphragm. The more dentin is formed in the apical foramen, the most the lumens of the pulp canals become narrower (due to the formation of parietal dentin).
Two strategies were used related to pulp therapies: either cell-based or cell-free therapies. The dental pulp includes a variety of cells contributing to dentin formation, pulp healing and to defense cells.
Pulp Cells
Fibroblasts and other cell lines such as dendritic cells, macrophages and T-lymphocytes are present within the dental pulp. Structural, growth factors-releasing cells, transcription factors and stem cells or induced stem cells are implicated in pulp healing, pulp re-formation and/or pulp regeneration.
Fibroblasts
Fibroblasts are resident cells found in great amount in the pulp tissue (stromal fibroblasts or pulpoblasts [1]) – the principal pulp cells. The function of fibroblast is to form and maintain collagen fibers and ground substance.
- In case of young teeth, the fibroblasts have abundant cytoplasm and organelles and actively synthetize extracellular matrix proteins. The fibroblasts are stellate-shaped cells having extensive processes that communicate with the processes of the other pulp fibroblasts to form a syncytium produced by intermittent communications between cells. Fibroblasts are linked by desmosomes, hemi-desmosomes, gap junctions and small tight junctions. The cells slide from the apex to the crown and underwent apoptosis in the coronal pulp, beneath the odontoblasts and the Höhl’s layers [2]. Population doubling time for pulp cells at passage 3 was 22.6 ± 0.5hours [3].
- In older pulp, the fibroblasts appear spindle or round, shaped with short processes and few cytoplasmic organelles. Fibroblasts help in the inflammatory and healing process by secretion of growth factors, transcription factors, cytokines, colony stimulating factors, FGF-2 and VEGF.
A variety of growth factors have successfully been used for dentin-pulp complex regeneration, including Transforming Growth Factors (TGFs), Bone Morphogenetic Proteins (BMPs), Platelet-Derived Growth Factor (PDGF), Insulin-Like Growth Factor (IGF) and Fibroblast Growth Factors (FGFs). BMP-2, BMP-4, BMP-7 have been shown to differentiate pulp cells into odontoblasts, making the BMP family the most likely candidate for dental clinic applications.
Human pulp fibroblasts from third molars express two crucial roles in pro-angiogenic factors, VEGF and FGF-2.
Stem Cells
Numerous types of stem cells have been obtained from dental tissue, including DPSCs, SHED, PDLSCs, SCAPs and Dental Follicle Cells (DFCs). All these cells can be used to regenerate the dental pulp. They are polyhedral shaped cells, larger than the fibroblasts, having a large oval nucleus, with peripheral processes. They may differentiate into microphage, odontoblast or fibroblast. They decrease in number as age increase.
BMMSCs express the Oct-4, Nanog, STRO-1, CD73, CD90, CD105, CD146 and are negative for CD14, CD34, CD45 and human leukocyte antigen-DR. Based on their multi-lineage differentiation potential and their high proliferative capacity, BMMSCs have a great potential for stem cell-based regenerative therapies. Similar to MSCs, DPSCs are positive for CD29, CD44, CD59, CD90, CD106 and CD146 and negative for CD34, CD45 and CD11b. DPSCs are described as a highly proliferative cell population with the self-renewal ability and multi-lineage differentiation potential.
DPSCs possess mesenchymal stem cell properties such as a fibroblast-like morphology, adherence to a plastic surface and the ability to form colonies when cultured in vitro. They differentiate into odontoblasts and neural-like cells under appropriate inductive conditions. Apical papilla means the soft tissue at the apices of developing SCAPs. They are residing in the apical papilla of incompletely developed teeth. SCAPs are responsible for the formation of dentin.
PDL stem cells, dental follicle precursor cells display potential capabilities during cellular therapies for periodontal diseases [4].
Defense Cells
They include mast cells, plasma cells, histocytes/macrophages. In addition, vascular cells are found, including neutrophils, eosinophils, basophils, lymphocytes and monocytes. These vascular cells emigrate from the blood vessels and developed a characteristic response to inflammation.
Mast Cells: These cells are found along blood vessels in inflamed pulps.
Plasma Cells: are seen during inflammation of the pulp and they are responsible for the production of antibodies. Histiocytes/macrophages or Mononuclear Phagocyte System (MPS)
B-lymphocytes are rarely encountered in the normal dental pulp.
T-lymphocytes are classified into Th1predominantly producing interleukin IL-2 and interferon-gamma (IFN- and Th2 cells (interferon-gamma (IFN-g).
Endothelial cells: The toll-like Receptor family TLR2 and TLR4 signaling and production of chemokines.
Chemokine [C-C Motif] may attract immune cells. Engagement of odontoblast led to nuclear factor-κB (NF-κB) and p38 Mitogen-Activated Protein Kinase (MAPK). Odontoblast-derived CXCL1, CXCL2 and CXCL8, known to attract neutrophils and CXCL10, attracting T-cells, could be involved in the accumulation of other populations of immune cells at the dentin-pulp interface.
Pulpal dendritic cells, composed of a group of hematopoietically derived cells with high dendritic morphology, expression of class II molecules, high motility, limited phagocytic activity and Antigen-Presenting Cells (APC) capacity. They activate T-lymphocytes [5]. The characteristics of several immunocompetent cells that are now considered to be essential for the induction of antigen-specific reactions in the dental pulp because of their critical role in pulpal immunosurveillance recognized. Immunohistochemical analyses have demonstrated the presence of two types of accessory cells-1-one with a dendritic morphology located in the periphery of the pulp and 2-one with a macrophage-like appearance located more centrally.
iPS: Viral introduction of 4 transcription factors may be used for pulp regeneration [6].
Other Cells
Innervation. Nerve plexus of Raschkow are located in the cell-rich zone. The two main sensory nerve fibres are:
Myelinated A-fibres / A-delta 90% of the A-fibres 10% are A-beta fibres. Transmission of pain is faster than with C-fibres.
Unmyelinated C-fibres they extend in the odontoblastic layer. The C-fibre stimulation result in a slow pain.
Myelinated axons are characterized by the nodes of Ranvier which allow saltatory conduction, increasing the speed of signal conduction.
Vascularization: Arterioles, veins.
Successive Glomerules feed limited areas, about 100-150 μm wide in the crown.
All of the main blood vessels and lymph drainages of dental pulp pass though the tooth root apexes, forming a fishnet which make the apex the main pathway for tooth nutrition and waste exchange. In some teeth, there are also much smaller openings of lateral canals, located near the apical foramen.
Lymphatic Circulation: Endothelial cells are implicated in immune defense. Present in the apically located periodontal lymphatic vessels. Lymph capillaries originated as blind sacs in the odontoblast layer drained collecting vessels, passes through the roots and merge into large single vessels. Injected in the pulp horn with colloidal carbon, the teeth extracted 1- to -3 hours later were characterized by a thin endothelium with intercellular clefts, the basement membrane being absent or incomplete. Pericytes were lacking. No red blood cells were visible [7].
Pericytes (Rouget Cells): are precursors that generate mature mesenchymal cells that differentiate into osteoblasts, odontoblasts, myoblasts and adipocytes in-vitro. Molar pericytes expressed 3,980 genes not detected in incisor pericytes. Molar and incisor pericytes are similar but not identical in function. Preodontoblasts may originate from perivascular cells migrating out of capillary walls into the surrounding fibrous pulp tissue. Indirect effect of the Bmp2 gene are effective in odontoblasts on formation of the vascular bed and associated pericytes in the pulp. This vascular niche and numbers of CD146+ pericytes are likely controlled by odontogenic and Bmp2-dependent VEGF-A production in odontoblasts. The complex roles of Bmp2, postulated to be both direct and indirect, lead to permanent defects in the teeth throughout life and result in teeth with low quantities of dentin and dentin of poor quality [8] (Fig. 1-4).
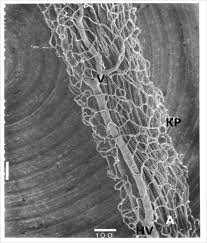
Figure 1: Root vascular network forming a fishnet network.
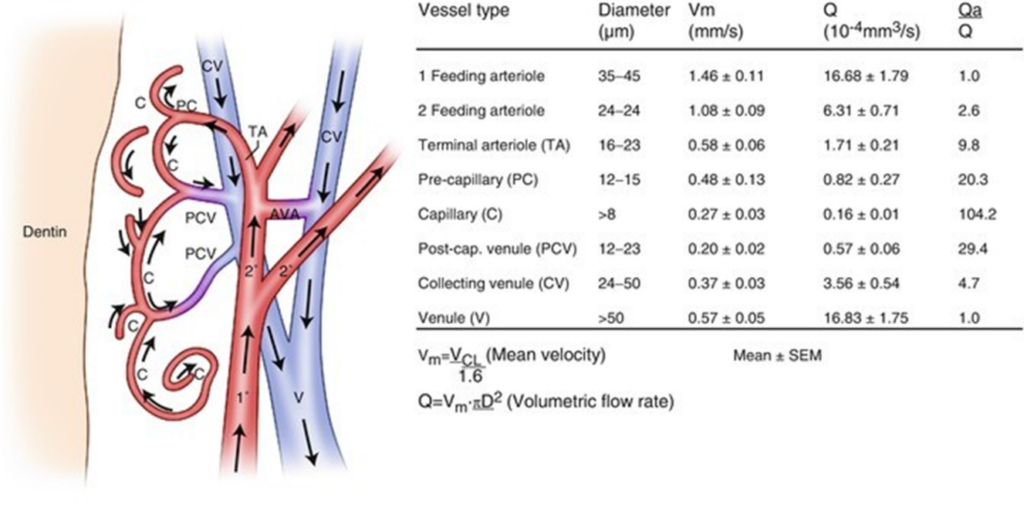
Figure 2: In the crown, feeding arterioles and collecting venules fed areas about 100-150 mm wide.
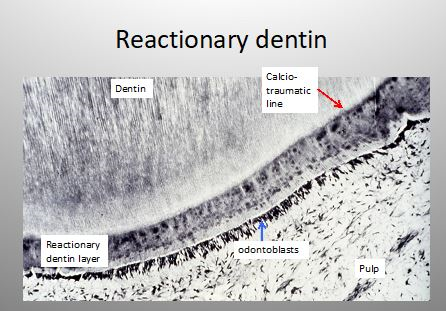
Figure 3: Reactionary dentin under a calciotraumatic line.
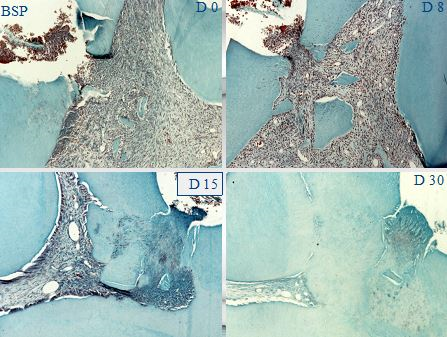
Figure 4: BSP implantation in a pulp exposure gradually field by reparative dentin that occlude the pulp exposure. After 30 days, the pulp is totally closed by tertiary dentin.
Pulp Capping
The initial effect of Ca(OH)2 is the development of a three-layer necrosis. This stimulate slight irritation and stimulate the pulp defense and repair starting with vascular and inflammatory cell migration. This is followed by the repair process, including migration and proliferation and formation of collagen. The mineralization is then initiated [9].
Solutions including sodium hypochlorite can be used as cleaning agents [10].
Indirect Capping involves dentin-binding agent, calcium hydroxide and others capping biomaterials. Calcium hydroxide was introduced by Hermann in 1920 [11], described by Zander and Glass in 1939 [12] (see review by Goldberg [13]).
Indirect pulp treatment is recommended to heal deep carious lesions, but have no signs or symptoms of degeneration. Using calcium hydroxide as liner, after 2 years 83% of success was obtained. Beneath a dense calciotraumatic line, tubular or atubular reactionary dentin is formed. This dentin is developed in reaction to transdentinal stimulation of the odontoblasts.
Direct pulp capping: When the pulp of a healthy pulp is inadvertently exposed during an operative procedure, Ca(OH)2 is placed directly over the exposure and reparative dentine formation is stimulated. Direct pulp capping by calcium hydroxide leads to the formation of a dentin bridge the odontoblasts-like cells occluding the pulp exposure by reparative dentin [14].
The successes of direct pulp capping are less than indirect capping, decreasing to 37% after 5 years and 13% after 10 years, producing a bone-like structure, made of collagen and non-collagenous proteins (namely osteocalcin and osteopontin). Due to the presence of osteocytes inside bone lacunae this bone is also called osteodentin [15].
Pulp dis-infection: The published data recommend clindamycin dose. It was reported that the association spiramycin-metronidazole at the usual dosage fails to cover the full bacterial spectrum in infections. Amoxicillin-clavulanic acid, clindamycin and moxifloxacin are the antibiotics of choice. A polymicrobial flora has been described in odontogenic infections, with strict anaerobes and a relatively limited microbial spectrum. Penicillin VK, 500 mg, 4 times a day is the first choice antibiotic prescribed. Cindamycin 150 mg, 4 time a day. Clindamycin and erythromycin were also prescribed together during a systematic exposure, the average duration being 6.80 to 7.58 days. Azithromycin was also recommended, but at very low doses.
Treatment of non-vital teeth: pulp regeneration
There are two possibilities:
- The pulp is reversibly inflamed and the remnant tissue serves as a source of resident stem cells.
- The pulp, irreversibly inflamed or necrotic, has to be completely removed and following chemo-mechanical cleaning methods, SCAP may proliferate and recolonize gradually the root. This less invasive situation is the cell-free scaffold dis to deliver bioactive cues, promoting disinfection of the tooth in order to recruit the remaining stem cells that differentiate into functional odontoblasts [16-19].
In a very few cases, a limited number of stem cells may be obtained from a dental tissue. Pluripotent Stem Cells (iPSC) were induced by viral introduction of four transcription-factors mediating reprogramming (Oct4, Sox2, Klf4 and c-Myc). Human or animal Stem Cells include DCSC, SHEDs, SCAP, PDLSC and DFPSC.
Apexification that is occurring when the pulp is non-vital, infected or not, enhanced the continued root dentin formation inside the lumen, linked with apical closure and possibly with radicular dental pulp regeneration. After laceration of the periapex with a file until bleeding occurs, it provides a matrix of blood clot into which cells could grow. Dressing with a triple antibiotic paste, consisting of metronidazole, ciprofloxacin and minocycline, allows apexification.
- Metronidazole exhibits a broad spectrum of activity. It binds to the DNA, disrupting its helical structure and leads to rapid cell death
- Tetracyclines, which include doxycycline and minocycline, are effective against most spirochetes and many anaerobic and facultative bacteria. They act by inhibiting protein synthesis. Minocycline is a derivative of tetracycline. It is available in many topical forms ranging from gel mixtures to sustained release of microspheres
- Ciprofloxacin has a bactericidal mode of action. Ciprofloxacin has very potent activity against gram-negative pathogens but a limited activity against gram-positive bacteria. Most anaerobic bacteria are resistant to ciprofloxacin. Side effects of ciprofloxacin have been reported. It was found that the drug is clinically safe when applied in low doses, adverse side effects should be minimized
Conclusion
For a necrotic and infected dental pulp, after disinfection using a triple-antibiotic therapy, the radicular pulp may regenerate and restore most of its activities. Apical papilla cells may proliferate and recolonize the dental pulp. Parietal dentin thickening and/or apexification and apical closure associated with pulp regeneration [15].
Conflict of Interest
The author declares no conflict of interest.
References
- Baume LJ. The Biology of pulp and dentine. Monographs in Oral Science. 1980;8(1):220.
- Hirata A, Dimitrova-Nakov S, Djole S-X, Ardila H, Baudry A, Kellermann O, et al. Plithotaxis, a collective cell migration, regulate the sliding of proliferating pulp cells located in the apical niche. Connect Tissus Res. 2014;55(S1):68-72.
- Huang G T-J, Shagramanova K, Chen SW. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in-vitro. J of Endod. 2006;12(11):1066-73.
- Li Y, Shu L-H, Yan M, Dai W-Y, Li J-J, Zhang G-D, et al. Adult stem cell-based apexogenesis. World J Methodol. 2014;4(2):99-108.
- Jontell M, Okiji T, Dahlgren U, Bergenholtz G. Immune defence mechanisms of the dental pulp. Crit Rev in Oral Biol & Medicine. 1998;9(2):179-200.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-76.
- Frank RM, Wiedemann P, Fellinger E. Ultrastructure of lymphatic capillaries in the human dental pulp. Cell & Tissue Research. 1977;178:229-38.
- Yang W, Harris MA, Cui Y, Mishina Y, Harris SE, Gluhak-Hzinrich J. BMP2 is required for odontoblast differentiation and pulp vasculogenesis. J Dent Rec. 2012;91(1):58-64.
- Schröder U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J Dent Res. 1985;64:541-48.
- Hilton TJ. Keys to clinical success with pulp capping: a review of the literature. Oper Dent. 2009;34(5):615-25.
- Hermann BW. Calciumhydroyd als mittel zum behanein und fullen von zahnwurzelkanlen. Wurzburg Med Diss. 1920:29.
- Zanders HA, Glass RL. The healing of phenolized pulp exoposures. Oral Surg Oral Med Oral Radiol Endod. 2005;100(2Suppl):S97-S101.
- Goldberg M. Indirect and direct pulp capping: reactionary vs reparative dentins. JSM Dent. 2020;8(1):1119.
- Gary A. Direct and indirect pulp capping: a brief history, material innovations, and clinical case report. Compend Contin Educ Dent. 2018;39(3):182-9.
- Cox CF, Sübay RK, Ostro E. Suzuki S Tunnels defects in dentin bridges: their formation following direct pulp capping. Operative Dentistry. 1996;21(1):4-11.
- Huang GTJ, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla:the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34(6):645-51.
- Friedlander LT, Cullinan MP, Love RM. Dental stem cells and their potential role in apexogenesis and apexification. Internat Endod J. 2009;42:955-62.
- Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J of Endod. 2008;34(2):166-71.
- Nosrat A, Li KI, Vir K, Hicks ML, Fouad AF. Is pulp necessary for root maturation. J Endod. 2013;39:1291-5.
This work is licensed under a Creative Commons Attribution 2.0 International License.


