Exploring the Intricacies of Encephalitis in Mosquito-Borne Diseases through Vector-Host-Pathogen Interactions: A Critical Review
Mohammad Jamali1*, Syed Mahmood Shahidul Islam2, Jamilur Rahman Bhuiyan3, Calvin R Wei4, Sadia Afrin5, Peter Singh6, Zahra Musarrat7, Prosenjit Rajbongshi8, Timothy Singh9, Rezwan Ahmed Mahedi5,10, Nikolaos Syrmos11, Lemar Cardenas de Guia12
1Assistant Professor, Faculty of Medical and Health Sciences, Liwa College, Al Meryal Campus Al Ain, Abu Dhabi
2Divisional Health and Safety Officer (South Asia and Central Asia); SMEC International Pty Ltd
3Department of Pharmacy, Jahangirnagar University, Bangladesh
4Department of Research and Development, Shing Huei Group, Taipei, Taiwan
5Department of Pharmacy, Comilla University, Cumilla, Bangladesh
6School of Pharmacy, Brac University, Bangladesh
7Department of Pharmaceutical Sciences, North South University, Bangladesh
8Pharmacy Discipline, Khulna University, Bangladesh
9University of Huddersfield, Department of Pharmacy, Queens Gate, Huddersfield, West Yorkshire, England
10Research Secretary, Bangladesh Pharmacists’ Forum (Comilla University), Bangladesh
11Aristotle University of Thessaloniki, Thesaaloniki, Macedonia, Greece
12Curry Elementary School District of Sta Margarita II Division of Samar, Philippines
*Correspondence author: Mohammad Jamali, Assistant Professor, Faculty of Medical and Health Sciences, Liwa College, Al Meryal Campus Al Ain, Abu Dhabi; Email: mjamali68@gmail.com
Published Date: 31-12-2023
Copyright© 2023 by Jamali M, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
| Received 05 Dec, 2023 | Accepted 24 Dec, 2023 | Published 31 Dec, 2023 |
Abstract
Environmental changes, medication resistance and sociodemographic shifts have all contributed to a dramatic increase in vector-borne diseases in the last 40 years, impacting both people and domestic animals. Pandemics like the Dengue fever epidemic that hit Bangladesh in 2023 show how devastating these illnesses may be on a global scale. The authors of this work stress the significance of comprehending vector-host-pathogen pathways via their examination of arboviruses in Asia. The ecology and biology of Culex, Culiseta and Aedes species in connection to Dengue Virus, Japanese Encephalitis (JE), Eastern Equine Encephalitis (EEE) and Western Equine Encephalitis (WEE) are explored in a thorough literature review that utilizes MeSH terminology. Beginning with the Japanese Encephalitis Virus (JEV), this article examines the virus’s transmission from insects to vertebrates and, inadvertently, to humans as a disease. We then go on to dengue encephalitis, breaking its intricate pathophysiology into parts. This includes aspects like immune-mediated consequences, systemic problems and direct invasion. The Aedes mosquito is a key player in the human-mosquito-human cycle that transmits Dengue Virus (DENV) and the extrinsic incubation time impacts outbreaks. Continuing to focus on the neuroinvasive effects on horses and people, we have Eastern Equine Encephalopathy (EEE). The interaction between birds and Culiseta melanura mosquitoes highlights the transmission of the enzootic cycle. At last, we look at Western Equine Encephalitis (WEE), which WEEV causes and how it affects both horses and people. Importantly, Culex species, which include mosquitoes and birds, serve as vectors in the enzootic cycle. Factors including climate change and international travel are included in the study’s conclusion, which emphasizes the significance of continuing research to monitor and reduce the worldwide effect of these arboviruses.
Keywords: Dengue Virus; Japanese Encephalitis (JEE); Eastern Equine Encephalitis (EEE); Western Equine Encephalitis (WEE); Viral Transmission
Article Type
Review Article
Introduction
The last four decades have seen a tremendous increase in the incidence of vector-borne illnesses that may infect both people and domestic animals. Typically, the primary causes are alterations in the natural environment (such as deforestation, new dams and irrigation systems, etc.), medicine resistance and sociodemographic changes (such as urbanization, increased population density, substandard housing, etc.). However, sudden and unexpected emergencies like the Dengue fever pandemic in Bangladesh in 2023 are not unheard of. A rise in international commerce and travel has made these catastrophes more probable. Although such emergencies are still very difficult to foresee, compiling a list of prospective arbovirus vectors in Asia can help with risk prediction. Previously geographically isolated illnesses have expanded throughout the globe, leading to an increase in the prevalence of diseases transmitted by mosquitoes [1]. When it comes to the transmission and dissemination of arboviruses, such as dengue, West Nile Virus (WNV), Eastern Equine Encephalitis (EEE) and Western Equine Encephalitis (WEE), vector-host-pathogen interactions play a crucial stage in the process. Vectors are necessary to transfer these viruses from one host to another. In the case of dengue and West Nile virus, mosquitoes are the most common vectors, whereas Culicoides midges are responsible for EEE and WEE. Several molecular and ecological interactions occur during the delicate dance between the vector, the host and the pathogen. While feeding on an infected host, often a person or a bird, mosquitoes may get infected. After that, the virus passes through a complicated chain of internal processes inside the mosquito, finally reaching the salivary glands. When the infected mosquito bites a new host, it injects the virus into the bloodstream, the first step in the infection process. Several elements influence whether or not viral transmission is successful. These include the host’s immunological response, the vector’s competence and environmental conditions [2]. To create successful measures to prevent the spread of these arboviruses and reduce the impact of related illnesses on human and animal populations, it is essential to have a thorough understanding of the intricacies of these interactions.
Methodology
We performed a comprehensive literature review. By using MeSH terms like “Dengue Virus”, ” Japanese Encephalitis (JEE),” “Eastern Equine Encephalitis (EEE),” “Western Equine Encephalitis (WEE),” and “Viral Transmission,” we searched credible resources like PubMed databse. The goal of this work package is to compile the most up-to-date research on the ecology and biology of the Culex, Culiseta and Aedes species as they relate to the transmission of Dengue Virus, Japanese Encephalitis (JEE), Eastern Equine Encephalitis (EEE), Western Equine Encephalitis (WEE).
Vector-Host-Pathogen Mechanism
Japanese Encephalitis Virus (JEV)
A major public health issue, Japanese Encephalitis (JE), is caused by the flavivirus known as JEV, which is mostly spread by mosquitoes. An underappreciated tropical illness, the virus claims a disproportionately high number of lives annually in East Asia [5]. Flaviviruses, such as JEV, may spread rapidly due to the nature of their insect vectors, the prevalence of metropolitan areas and the ease of international travel [7]. According to Pujhari, et al., the virus has formed a complicated web with the help of insects, vertebrates as reservoirs and unintentional hosts like humans [8]. Also, some areas, like Cambodia, have mosquitoes that might spread JEV and other arboviruses [4]. In addition to affecting humans, the virus multiplies in vulnerable hosts such as pigs, ducks and chickens, meaning its effects go beyond only human health [3]. As a top cause of viral encephalitis in Asia, it is essential to understand the ecology and evolution of JEV [6]. Sanborn et al. noted that the dynamics of the virus’s transmission and possible control methods may be better understood via the continuing investigation of the virus’s virome diversity in mosquitoes. JEV transmission is complex and involves vectors, hosts and environmental variables thus, it’s important to avoid and manage it using comprehensive tactics [9].
Species | Presence | Vector Status | Reference |
Culex Pipiens | Proven vector of WN virus | [58, 59] | |
Potential vector of RVF virus (virus isolation, vector competence studies) | [60] | ||
Potential vector of JE virus (vector competent studies) | [61] | ||
Culex Modestus | Palearctic distribution | Proven vector of WN virus | [62, 63] |
Culex Theileri | Presence in some parts of Europe | Proven vector of WN virus in South Africa | [64] |
Potential vector of RVF virus (isolation and laboratory studies) | [65] | ||
Aedes Albopictus | Presence in some parts of Europe | Proven vector of dengue virus | [66] |
| Suspected vector of VEE viruses (vector competence studies) | [67] | |
Potential vector of EEE virus (isolation and laboratory studies) | [68] | ||
| Potential vector of WN virus (isolation and laboratory studies) | [69] | |
Suspected vector of JE virus (natural infection) | [70] | ||
Aedes Vittatus | Western Mediterranean distribution | Potential vector of Dengue virus (Experimental transmission, natural infection) | [71] |
Aedes Dorsalis | European boreal distribution | Potential vector of WEE virus (experimental transmission, natural infection) | [72] |
Aedes Vexans | Whole Europe | Suspected vector of RVF virus (natural infection) | [73] |
Aedes Caspius | Whole Europe | Potential vector of RVF virus (experimental transmission) | [74] |
Table 1: Suspected vector species for some arboviroses in European countries.
Transmission of Japanese Encephalitis Virus (JEV): A flavivirus, Japanese encephalitis (JE) has a strong kinship with St. Louis and West Nile encephalitis viruses. Bite vectors of the Culex mosquito family, especially Culex tritaeniorhynchus, transmit the JE virus to humans. It is widely believed that Culex and maybe some Aedes mosquitoes are responsible for transmitting JEV from wild reservoir hosts, such as Ardeid birds, or amplifying hosts, such as farmed pigs, to people. But JEV can still circulate in places where Ardeid birds and domestic pigs aren’t very common, like Singapore, where sentinel poultry is monitored for the virus long after pig production has ended. Different regions may thus have different JE epidemiology [11,12]. Several Asian regions have demonstrated that domestic ducks and poultry are susceptible to JEV [13-16]. A serosurvey in the Cambodian province of Kandal revealed that the infectious force of JEV, or the probability of infection at any given moment, was similar to that experienced by pigs [14]. In addition to developing considerable viremia after being bitten by infected mosquitoes, young ducklings and chicks may be able to infect mosquitoes that feed on them [17-22]. This suggests that young fowl may be competent hosts to transmit JEV to vectors [23]. But no one has looked at chickens as potential natural hosts for JEV in any detail just yet.
Dengue Encephalitis
According to Baheti, et al., dengue encephalitis is an uncommon but serious side effect of dengue virus infection that results from the virus directly invading the central nervous system [20]. Dengue encephalitis has a complicated pathophysiology that incorporates several different mechanisms. According to research, there are three main pathogenic mechanisms for the neurological consequences of dengue infection: the virus’s direct invasion of the central nervous system, which results in neurotropic effects like meningitis and encephalitis; systemic complications that lead to encephalopathy and stroke; and post-infectious immune-mediated complications that include acute disseminated encephalomyelitis and Guillain-Barre syndrome [23,24,27]. Furthermore, several individuals with neurological symptoms discovered the virus in their cerebrospinal fluid, suggesting that it directly affects the central nervous system [21,22,25,26]. Emphasize the significance of comprehending vector-host-pathogen interactions in dengue encephalitis and the function that mosquito vectors play in transmitting dengue virus and other flaviviruses. These results demonstrate the complex pathophysiology of dengue encephalitis, which includes host defense mechanisms, viral virulence factors and the function of mosquito vectors in virus transmission.
Transmission of Dengue Virus (DENV): Infected female mosquitoes of the genus Aedes, particularly Aedes aegypti and Aedes albopictus, are the most common vectors for the transmission of dengue fever [28]. But in other parts of the globe, the other two vectors, such Aedes polynesiensis and Aedes niveus, are the ones that serve as secondary vectors [29]. It is believed that DENV transmission occurs in a human-mosquito-human cycle. In this case, mosquitoes get the infection by feeding on an infected person’s blood. DENV replicates in the mosquito’s midgut for three to five days before moving on to the salivary gland, which may take up to fourteen days. The adult virus may then infect people who are vulnerable to it by biting an infected mosquito. The anticipated duration between when a mosquito becomes sick and when it may spread the disease, known as the Extrinsic Incubation Period (EIP) is about 5 to 14 days. A crucial factor in determining the severity of dengue epidemics is that the EIP affects the percentage of infected mosquitoes that survive to spread the disease [30-32]. Despite the wide variation in parameter estimates for the EIP, this trait makes it suitable for modeling dengue transmission patterns [33].
After a mosquito bite, the virus travels through the blood and starts to multiply in cells that the virus has identified as potential hosts, such as monocytes and dendritic cells in the skin. Endothelial cells, hepatocytes and other cells are all subject to the virus’s effects, which include immunological abnormalities, cytokine overproduction and cell death [36]. According to Martins, et al., this causes signaling pathways to start the apoptotic process, which adds to the severity of the illness [35-39]. In addition, the virus hinders routes for thymidine production, which impacts the replication of the virus [34]. Functioning autophagy components also make it easier for viruses to replicate their RNA and produce infectious viruses [40,41]. After infecting certain immune cells, the virus travels to lymph nodes to replicate. Variegated cytopathies manifest in different degrees of severity after viral infection of various liver cells [37]. The first stage of dengue infection is a fever and the second stage is severe dengue, sometimes called Dengue Hemorrhagic Fever (DHF) or Dengue Shock Syndrome (DSS), which may be fatal. Different serotypes of the virus may cause secondary infections, which can increase the severity of the illness via antibody-dependent enhancement [35,37,38]. All of these results point to the complex ways in which the Dengue virus causes illness by infecting and controlling host cells (Fig. 1).
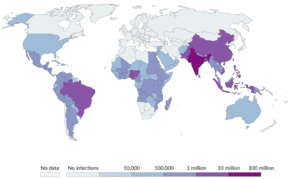
Figure 1: Recent distribution of dengue virus in the whole world.
Eastern Equine Encephalitis
The virus known as Eastern Equine Encephalitis (EEE) causes a destructive form of arboviral neuroinvasive illness in horses. Survivors of this disease often have severe neurological sequelae and a high death rate [39,40]. Among neuroinvasive arboviral infections in the US, EEEV has the greatest case-fatality rate. Animals and people alike are susceptible to the virus’s devastating encephalitis, which may lead to fatalities or lasting neurological consequences [47,53]. During the transmission season, outdoor activities near forested wetlands have been linked to the illness marked by localized radiography symptoms. There is strong evidence that EEEV is highly pathogenic to horses and people and it has been suggested that outbreaks in the northeastern United States may originate in Florida [44]. Multiple U.S. states have seen infections due to the virus. The epidemiology of the virus has been investigated by using the persistence of antibodies to EEEV in birds [42]. Using GIS and spatial statistics, researchers in Florida have examined the geographical epidemiology of EEE [44]. According to Sah et al., our results highlight the importance of EEE as a public health problem and the need for ongoing monitoring and mitigating methods to combat this fatal zoonotic virus transmitted by mosquitoes.
Transmission of Eastern equine encephalitis Virus (JEV): Birds and Culiseta melanura mosquitoes, which lay their eggs in freshwater hardwood swamps, are the main vectors in the enzootic cycle that transmits Eastern equine encephalitis (EEE) [46]. This cycle is crucial for the virus’s maintenance and mosquitoes, especially Aedes sollicitans, are a major vector for EEE transmission to horses and birds [50]. The virus is very contagious and may spread by airborne particles [45]. The increase in EEE occurs at the same time as the virus’s vectors and the main hosts, which are birds [41,42]. Also, the virus may make people sick with encephalitis, which can lead to death or permanent brain damage in those who have it [47]. Savage, et al., state that the American Robin and Common Grackle are the most significant reservoir hosts for the virus according to the EEEV transmission model [48]. In addition, the virus has the ability to infect macrophages and dendritic cells, which affects its pathogenesis [43]. Elevated mortality rates among equine and human cases of EEE highlight the seriousness of the disease [44]. In general, a web of relationships exists between birds, mammals and mosquitoes that allows the EEE virus to spread and infect hosts, ultimately leading to severe cases of encephalitis [46].
Bangladesh | 2022 | 2021 | 2020 | 2019 | 2018 |
Japanese Encephalitis | 82 | 82 | 32 | 86 | 96 |
Dengue | 62,382 | 28,249 | 1,193 | 1,01,354 | 10,148 |
India | |||||
Japanese Encephalitis | 1271 | 489 | 718 | 2496 | 1707 |
Dengue | – | 1,64,103 | 44,585 | 2,05,243 | 1,24,493 |
United State | |||||
Dengue | 1,247 | 205 | 354 | 1,474 | 483 |
Eastern Equine Encephalitis | – | 112 | 142 | 184 | 107 |
West Nile Virus | 1,126 | 2,911 | 731 | 971 | 2,647 |
La Crosse Encephalitis | 22 | 40 | 88 | 55 | 86 |
Saint Louis Encephalitis | 33 | 17 | 16 | 17 | 8 |
Table 2: Statistical report of JEV, DENV, EEV, WEEV of last five year (2018-2022) [75].
Western Equine Encephalitis (WEE)
Western Equine Encephalitis (WEE) is a serious neurological disease caused by the Western Equine Encephalitis Virus (WEEV), a member of the Alphavirus genus. WEEV is transmitted to humans and horses by mosquitoes and is known to cause lethal encephalitis in both species [50]. The virus is primarily found in North and South America. It is closely related to other New World alphaviruses, such as the Eastern Equine Encephalitis Virus (EEEV) and the Venezuelan Equine Encephalitis Virus (VEEV) [51]. WEEV, EEEV and VEEV are considered naturally emerging pathogens and potential biological weapons, posing a significant public health threat. The virus is known to persist in the environment, with strains remaining genetically unchanged for long periods, indicating local persistence. The enzootic cycle of WEEV involves birds and mosquito vectors, with birds serving as primary hosts for the virus [46]. The virus has been the cause of multistate outbreaks in the United States, highlighting its potential for widespread impact. Surveillance and research on WEEV have been conducted using various animal models, including house finches and cotton rats, to understand its transmission dynamics and pathogenesis [56]. Additionally, studies have explored the potential for antiviral treatments against WEEV, indicating the ongoing efforts to develop therapeutic interventions for this disease.
Transmission of Western Equine Encephalitis Virus (JEV): According to Baxter and Heise (2018), the virus that causes Western Horse Encephalitis (WEE) is the Western Equine Encephalitis Virus (WEEV), an alphavirus that is common throughout the Americas [50, 51]. Mosquitoes and birds keep WEEV in an enzootic cycle; humans and horses are considered dead-end hosts [54]. Animals and people alike are susceptible to the virus’s devastating encephalitis, which may lead to neurological complications or even death [46]. There are several viruses in the Venezuelan equine encephalitis complex. However, the principal enzootic vectors of WEEV are species of the subgenus Culex (Melanoconion) Theobald [57]. The virus has a tight relationship with two other potentially lethal equine encephalitis viruses—the Eastern Equine Encephalitis Virus (EEEV) and the Venezuelan equine encephalitis virus (VEEV) [53]. Most West African enzootic viral transmission occurs in an enzootic cycle involving mosquitoes and birds, with birds acting as the virus’s principal amplifying hosts [52]. Multiple bird species and mosquitoes are involved in the virus’s role in epizootic transmission cycles [56]. When controlling and monitoring WEEV and similar arboviruses, knowing how the virus spreads and which hosts and vectors are involved is vital (Fig. 2).
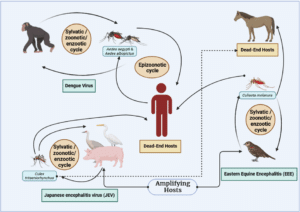
Figure 2: A visual representation of various kind of mosquito borne diseases from animal to human [46-56].
Conclusion
In conclusion, the emergence and spread of vector-borne diseases, such as those caused by arboviruses like the Japanese encephalitis virus (JEV), the Dengue virus (DENV), the Eastern Equine Encephalitis Virus (EEEV) and the Western Equine Encephalitis Virus (WEEV), present significant challenges to the public health of the entire world. Regarding the transmission cycles of these viruses, the delicate dance between vectors, hosts and pathogens highlights the need to grasp the processes under the surface fully. To contribute to this knowledge, this extensive literature review aimed to investigate the vector-host-pathogen relationships for JEV, DENV, EEEV and WEEV. Some ecological, molecular and environmental variables play a role in the transmission dynamics of these viruses. These dynamics entail the complicated connections between mosquitoes, birds, animals and humans. To develop effective preventative and control measures, it is essential first to identify the primary vectors, hosts and transmission patterns. Based on these results, it is clear that continued study is necessary to monitor and reduce the influence of arboviruses on human and animal populations. A proactive and multidisciplinary strategy is necessary to handle the complex difficulties presented by vector-borne illnesses and minimize the possible catastrophic implications of these diseases. This is because global variables such as climate change, urbanization and international travel are continuing to develop.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- Lee H, Halverson S, Ezinwa N. Mosquito-borne diseases. Prim Care. 2018;45(3):393-407.
- Mahmud F, Mahedi MR, Afrin S, Haque R, Hasan MS, Sum FA, et al. Biological and Insecticidal Effect of Citronella Oil: A Short Review. Clin Medicine and Health Res J. 2022;2(6):261-5.
- Adi AA, Astawa NM, Damayanti PA, Kardena IM, Erawan IG, Suardana IW, et al. Seroepidemiological evidence for the presence of Japanese encephalitis virus infection in ducks, chickens, and pigs, Bali-Indonesia. Bali Med J. 2016;5(3):533-7.
- Boyer S, Marcombe S, Yean S, Fontenille D. High diversity of mosquito vectors in Cambodian primary schools and consequences for arbovirus transmission. PLoS One. 2020;15(6):e0233669.
- Le Flohic G, Porphyre V, Barbazan P, Gonzalez JP. Review of climate, landscape, and viral genetics as drivers of the Japanese encephalitis virus ecology. PLoS Neglected Tropical Diseases. 2013;7(9):e2208.
- Mulvey P, Duong V, Boyer S, Burgess G, Williams DT, Dussart P, et al. The Ecology and Evolution of Japanese Encephalitis Virus. Pathogens. 2021;10:1534.
- Pierson T, Diamond M. The continued threat of emerging flaviviruses. Nature Microbiol. 2020;5(6):796-812.
- Pujhari SK, Prabhakar S, Ratho RK, Modi M, Sharma M, Mishra B. A novel mutation (S227T) in domain II of the envelope gene of Japanese encephalitis virus circulating in North India. Epidemiol Infect. 2011;139(6):849-56.
- Sanborn MA, Wuertz KM, Kim HC, Yang Y, Li T, Pollett SD, et al. Metagenomic analysis reveals Culex mosquito virome diversity and Japanese encephalitis genotype V in the Republic of Korea. Molecular Ecol. 2021;30(21):5470-87.
- Transmission of Japanese Encephalitis virus. Cdc.gov. 2022. [Last accessed on: December 24, 2023]
https://www.cdc.gov/japaneseencephalitis/transmission/index.html
- Lord JS, Gurley ES, Pulliam JRC. Rethinking Japanese encephalitis virus transmission: a framework for implicating host and vector species. PLoS Negl Trop Dis. 2015;9(12):e0004074.
- Bae W, Kim JH, Kim J, Lee J, Hwang ES. Changes of epidemiological characteristics of Japanese encephalitis viral infection and birds as a potential viral transmitter in Korea. J Korean MedSci. 2018;33(9).
- Adi AAAM, Astawa N, Ayu ADP, Made KI, Gusti MKEI, Suardana IW, et al. Seroepidemiological evidence for the presence of japanese encephalitis virus infection in ducks, chickens and pigs, Bali-Indonesia. Bali Medical J. 2016;5:189.
- Ladreyt H, Auerswald H, Tum S, Ken S, Heng L, In S, et al. Comparison of Japanese encephalitis force of infection in pigs, poultry and dogs in cambodian villages. Pathogens. 2020;9(9):719.
- Kalaiyarasu S, Mishra N, Khetan RK, Singh VP. Serological evidence of widespread West Nile virus and Japanese encephalitis virus infection in native domestic ducks (Anas platyrhynchos var domesticus) in Kuttanad region, Kerala, India. Comparative Immunol Microbiol Infect Dis. 2016;48:61-8.
- Pant GR, Lunt RA, Rootes CL, Daniels PW. Serological evidence for Japanese encephalitis and West Nile viruses in domestic animals of Nepal. Comparative Immunol Microbiol Infect Dis. 2006;29(2-3):166-75.
- Cleton NB, Bosco-Lauth A, Page MJ, Bowen RA. Age-Related susceptibility to Japanese encephalitis virus in domestic ducklings and chicks. Am J Trop Med Hyg. 2014;90(2):242-6.
- Di D, Li C, Zhang J, Hameed M, Wang X, Xia Q, et al. Experimental infection of newly hatched domestic ducklings via japanese encephalitis virus-infected mosquitoes. pathogens. 2020;9(5):371.
- Karna AK, Bowen RA. Experimental evaluation of the role of ecologically-relevant hosts and vectors in Japanese encephalitis virus genotype displacement. Viruses. 2019;11(1):32.
- Baheti G, Mehta V, Ramchandani M, Ghosh GC. Dengue fever with encephalitis: a rare phenomenon. Case Reports. 2018.
- Boyer S, Marcombe S, Yean S, Fontenille D. High diversity of mosquito vectors in Cambodian primary schools and consequences for arbovirus transmission. Plos One. 2020;5(6):e0233669.
- Carod-Artal F. Neurological manifestations of dengue viral infection. Research and Reports in Tropical Med. 2014;95.
- Ho JY, Liew YK, Loh J, Sohil P. Case report: Mononeuritis multiplex in the course of dengue fever. BMC Infect Dis. 2020;20:1-5.
- Kumar SS, Baitha U, Vyas S. An unusual case of Acute Motor Axonal Neuropathy (AMAN) complicating dengue fever. Drug Discover Therap. 2021;15(4):214-7.
- Onen H, Luzala MM, Kigozi S, Sikumbili RM, Muanga CJ, Zola EN, et al. Mosquito-borne diseases and their control strategies: an overview focused on green synthesized plant-based metallic nanoparticles. Insects. 2023;14(3):221.
- Sui L, Zhao Y, Wang W, Chi H, Tian T, Wu P, et al. Flavivirus prM interacts with MDA5 and MAVS to inhibit RLR antiviral signaling. Cell Biosci. 2023;13(1):9.
- Verma R, Sharma P, Garg RK, Atam V, Singh MK, Mehrotra HS. Neurological complications of dengue fever: Experience from a tertiary center of north India. Annals Ind Acad Neurol. 2011;14(4):272.
- Ferreira-de-Lima VH, Lima-Camara TN. Natural vertical transmission of dengue virus in Aedes aegypti and Aedes albopictus: a systematic review. Parasites Vectors. 2018;11:1-8.
- Zahoor MK, Rasul A, Zahoor MA, Sarfraz I, Zulhussnain M, Rasool R, et al. Dengue fever: a general perspective. Dengue Fever: A Resilient Threat in the Face of Innovation. 2019;1.
- Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7-16.
- Roy SK, Bhattacharjee S. Dengue virus: epidemiology, biology and disease aetiology. Can J Microbiol. 2021;67(10):687-702.
- Tjaden NB, Thomas SM, Fischer D, Beierkuhnlein C. Extrinsic incubation period of dengue: knowledge, backlog and applications of temperature dependence. PLoS Negl Trop Dis. 2013;7(6).
- Brady OJ, Johansson MA, Guerra CA, Bhatt S, Golding N, Pigott DM, et al. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasit Vectors. 2013;6:351.
- Fischer MA, Smith JL, Shum D, Stein DA, Parkins C, Bhinder B, et al. Flaviviruses are sensitive to inhibition of thymidine synthesis pathways. J Virol. 2013;87(17):9411-9.
- Garcia-Cordero J, Ramirez HR, Vazquez-Ochoa M, Gutierrez-Castaneda B, Santos-Argumedo L, Villegas-Sepulveda N, et al. Production and characterization of a monoclonal antibody specific for NS3 protease and the ATPase region of Dengue-2 virus. Hybridoma. 2005;24(3):160-4.
- Lei HY, Yeh TM, Liu HS, Lin YS, Chen SH, Liu CC. Immunopathogenesis of dengue virus infection. J Biomedical Sci. 2001;8:377-88.
- Lin YL, Liu CC, Lei HY, Yeh TM, Lin YS, Chen RM, et al. Infection of five human liver cell lines by dengue‐2 virus. J Med Virol. 2000;60(4):425-31.
- López-Lemus UA, Vásquez C, Vázquez-Campuzano R, Valle-Reyes S, Guzmán-Bracho C, Araiza-Garaygordobil D, et al. Dengue virus serotype 1 non-structural protein NS5 expression interferes with HIV replication in a CD4+ T-cell line. The Am JTropical Medicine and Hygiene. 2014;90(3):418.
- Martins SD, Silveira GF, Alves LR, Dos Santos CN, Bordignon J. Dendritic cell apoptosis and the pathogenesis of dengue. Viruses. 2012;4(11):2736-53.
- Mateo R, Nagamine CM, Spagnolo J, Méndez E, Rahe M, Gale Jr M, et al. Inhibition of cellular autophagy deranges dengue virion maturation. J Virol. 2013;87(3):1312-21.
- Reyes-del Valle J, Salas-Benito J, Soto-Acosta R, del Angel RM. Dengue virus cellular receptors and tropism. Curr Trop Medicine Rep. 2014;1:36-43.
- Calisher CH, Fremount HN, Vesely WL, El-Kafrawi AO, Mahmud MI. Relevance of detection of immunoglobulin M antibody response in birds used for arbovirus surveillance. J Clin Microbiol. 1986;24(5):770-4.
- Gardner CL, Burke CW, Tesfay MZ, Glass PJ, Klimstra WB, Ryman KD. Eastern and Venezuelan equine encephalitis viruses differ in their ability to infect dendritic cells and macrophages: impact of altered cell tropism on pathogenesis. J Virol. 2008;82(21):10634-46.
- Vander Kelen PT, Downs JA, Burkett-Cadena ND, Ottendorfer CL, Hill K, Sickerman S, et al. Habitat associations of eastern equine encephalitis transmission in Walton County Florida. J Med Entomol. 2012;49(3):746-56.
- Kumar B, Manuja A, Gulati BR, Virmani N, Tripathi BN. Suppl-2, M5: zoonotic viral diseases of equines and their impact on human and animal health. The Open Virol J. 2018;12:80.
- Lindsey NP, Staples JE, Fischer M. Eastern equine encephalitis virus in the United States, 2003–2016. The American J Trop Med and Hygiene. 2018;98(5):1472.
- Petrakova O, Volkova E, Gorchakov R, Paessler S, Kinney RM, Frolov I. Noncytopathic replication of Venezuelan equine encephalitis virus and eastern equine encephalitis virus replicons in Mammalian cells. J Virology. 2005;79(12):7597-608.
- Savage HM, Aggarwal D, Apperson CS, Katholi CR, Gordon E, Hassan HK, et al. Host choice and West Nile virus infection rates in blood-fed mosquitoes, including members of the Culex pipiens complex, from Memphis and Shelby County, Tennessee, 2002-2003. Vector-Borne and Zoonotic Dis. 2007;7(3):365-86.
- Trobaugh DW, Sun C, Bhalla N, Gardner CL, Dunn MD, Klimstra WB. Cooperativity between the 3’untranslated region microRNA binding sites is critical for the virulence of eastern equine encephalitis virus. PLoS Pathogens. 2019;15(10):e1007867.
- Walsh A, Glass G, Lesser, C, Curriero F. Predicting seasonal abundance of mosquitoes based on off-season meteorological conditions. Environmental and Ecological Statistics. 2007;15(3):279-91.
- Baxter V, Heise M. Genetic control of alphavirus pathogenesis. Mammalian Genome. 2018;29(7-8):408-24.
- Mahedi MR, Rawat A, Rabbi F, Babu KS, Tasayco ES, Areche FO, et al. Understanding the global transmission and demographic distribution of Nipah virus (NiV). Res J Pharm Technol. 2023;16(8):3588-94.
- Calvert AE, Bennett SL, Hunt AR, Fong RH, Doranz BJ, Roehrig JT, et al. Exposing cryptic epitopes on the Venezuelan equine encephalitis virus E1 glycoprotein prior to treatment with alphavirus cross-reactive monoclonal antibody allows blockage of replication early in infection. Virol. 2022;565:13-21.
- Chapman GE, Baylis M, Archer D, Daly JM. The challenges posed by equine arboviruses. Equine Veterinary J. 2018;50(4):436-45.
- Petrakova O, Volkova E, Gorchakov R, Paessler S, Kinney RM, Frolov I. Noncytopathic replication of Venezuelan equine encephalitis virus and eastern equine encephalitis virus replicons in Mammalian cells. J Virol. 2005;79(12):7597-608.
- Reisen WK, Lundstrom JO, Scott TW, Eldridge BF, Chiles RE, Cusack R, et al. Patterns of avian seroprevalence to western equine encephalomyelitis and Saint Louis encephalitis viruses in California, USA. J Medical Entomol. 2000;37(4):507-27.
- Williams MR, Savage HM. Identification of Culex (Melanoconion) species of the United States using female cibarial armature (Diptera: Culicidae). J Medical Entomol. 2009;46(4):745-52.
- Balenghien T, Vazeille M, Grandadam M, Schaffner F, Zeller H, Reiter P, et al. Vector competence of some French Culex and Aedes mosquitoes for West Nile virus. Vector-Borne and Zoonotic Dis. 2008;8(5):589-96.
- Esteves A, Almeida AP, Galão RP, Parreira R, Piedade J, Rodrigues JC, et al. West Nile virus in southern Portugal, 2004. Vector-Borne Zoonotic Dis. 2005;5(4):410-3.
- Hoogstraal H, Meegan JM, Khalil GM, Adham FK. The rift valley fever epizootic in Egypt 1977-1978 2. Ecological and entomological studies. Transactions of the Royal Society of tropical Medicine and Hygiene. 1979;73(6):624-9.
- Gresser I, Hardy JL, Hu SM, Scherer WF. Factors influencing transmission of Japanese B encephalitis virus by a colonized strain of Culex tritaeniorhynchus Giles, from infected pigs and chicks to susceptible pigs and birds. Am J Trop Medicine and Hygiene. 1958;7(4):365-73.
- Balenghien T. De l’identification des vecteurs du virus West Nile à la modélisation du risque d’infection dans le sud de la France (Doctoral dissertation, Université Joseph-Fourier-Grenoble I). 2006.
- Balenghien T, Fouque F, Sabatier P, Bicout DJ. Horse, bird and human-seeking behavior and seasonal abundance of mosquitoes in a West Nile virus focus of southern France. J Med Entomol. 2006;43(5):936-46.
- Mcintosh BM. Rift Valley fever. 1. Vector studies in the field. J S Afr Vet Med Assoc. 1972;43(4):391-5.
- McIntosh BM, Jupp PG, Dickinson DB, McGillivray GM, Sweetnam J. Ecological studies on Sindbis and West Nile viruses in South Africa. I. Viral activity as revealed by infection of mosquitoes and sentinel fowls. The South African J Med Sci. 1967;32(1):1-4.
- Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18(3):215-27.
- Smith DR, Carrara AS, Aguilar PV, Weaver SC. Evaluation of methods to assess transmission potential of Venezuelan equine encephalitis virus by mosquitoes and estimation of mosquito saliva titers. The Am J Trop Medicine and Hygiene. 2005;73(1):33-9.
- Turell MJ, Beaman JR, Neely GW. Experimental transmission of eastern equine encephalitis virus by strains of Aedes albopictus and A. taeniorhynchus (Diptera: Culicidae). J Med Entomol. 1994;31(2):287-90
- Tiawsirisup S, Platt KB, Evans RB, Rowley WA. A comparision of West Nile Virus transmission by Ochlerotatus trivittatus (COQ.), Culex pipiens (L.), and Aedes albopictus (Skuse). Vector-Borne Zoonotic Dis. 2005;5(1):40-7.
- George S, Jacob PG, Rao JA. Isolation of Japanese encephalitis & West Nile viruses from mosquitoes collected in Kolar district of Karnataka state during 1977-79. Indian J Med Res. 1987;85:235-8.
- Diallo M, Ba Y, Sall AA, Diop OM, Ndione JA, Mondo M, et al. Amplification of the sylvatic cycle of dengue virus type 2, Senegal, 1999–2000: entomologic findings and epidemiologic considerations. Emerging Infect Dis. 2003;;9(3):362.
- Fulhorst CF, Hardy JL, Eldridge BF, Presser SB, Reeves WC. Natural vertical transmission of western equine encephalomyelitis virus in mosquitoes. Science. 1994;263(5147):676-8.
- Fontenille D, Traore-Lamizana M, Diallo M, Thonnon J, Digoutte JP, Zeller HG. New vectors of Rift Valley fever in West Africa. Emerging Infect Dis. 1998;4(2):289.
- Turell MJ, Dohm DJ, Fernandez R, Calampa C, O’guinn ML. Vector competence of Peruvian mosquitoes (Diptera: Culicidae) for a subtype IIIC virus in the Venezuelan equine encephalomyelitis complex isolated from mosquitoes captured in Peru. J Am Mosq Control Assoc. 2006;22(1):70-5.
- Dengue – Bangladesh. Who.int. 2024. [Last accessed on: December 24, 2023]
https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON481
This work is licensed under a Creative Commons Attribution 2.0 International License.


