Mechanism of Action, Kinetics and a Bioactive Metabolites AM404 of Paracetamol
Sarbjeet Singh1, Mandeep Kaur2*
1- Research Scholar, Adesh Institute of Pharmacy and Biomedical Sciences, Bathinda, Punjab, India.
2- Assistant Professor, ISF College of Pharmacy, Moga, Punjab, India.
*Corresponding Author: Mandeep Kaur, Assistant Professor, ISF College of Pharmacy, Moga, Punjab, India;
E-mail: kaurm235@gmail.com
Citation: Kaur M, et al. Mechanism of Action, Kinetics and a Bioactive Metabolites AM404 of Paracetamol. J Clin Med Res. 2020;1(2):1-9.
Copyright© 2020 by Kaur M, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
| Received 15 Apr, 2020 | Accepted 02 May, 2020 | Published 09 May, 2020 |
Abstract
Paracetamol is that the one amongst the foremost broadly speaking NSAIDS used as antipyretic and analgesic drug within the world. However, still its Mechanism of Action (MOA) is not fully recognize. The potential Mechanism of Action (MOA) of paracetamol as its antipyretic and analgesic impact was thought multiple pathway. Some action relies on the inhibition of the Cyclooxygenases (COX enzyme) and a lot of recently study show that it additionally acts on serotonergic pathways (5HT pathway). A brand new metabolic pathway of paracetamol (as a prodrug) is come back to grasp, involving the generation of a lively matter, AM404 (N-4Hydroxyphenyl-5Z, 8Z, 11Z, 14Z-eicosatetraenamide), by the (FAAH) carboxylic acid organic compound hydrolase protein within the brain, was recently known. This review describes the theoretical knowledge that show the affiliation of this metabolic pathway within the analgesic & antipyretic action of acetaminophen (PCM) and its relationship with the enzyme protein (COX) and (5-HT) serotonergic systems. It additionally shows that the new systems (cannabinoid and vanilloid systems) and targets as well as the atomic number 20 channel receptor (Cav3.2), play an important role within the flightless bird of paracetamol.
Keywords
NSAID; Paracetamol; AM404; Para-aminophenol; Analgesic; Antipyretic; Pain; FAAH; CB1; TRPV1; Cav3.2
Introduction
Paracetamol is a Non-Steroidal Anti-Inflammatory Medicine (NSAIS) counselled for the first-line treatment of gentle to moderate pain and febrility. In line with British National Formulary (BNF), it is similar analgesic effectiveness like acetylsalicylic acid however while not medicament activity [1]. It is less irritant to the alimentary canal (GI track) than acetylsalicylic acid and, for this reason, it’s most used nonsteroidal anti-inflammatory, for older individuals. The one and solely reason to paracetamol is hypersensitivity to the Active Ingredient (AI) or excipients (Fig. 1) [2]. The warnings and precautions like non-cirrhotic alcoholic disease and severe excretory organ or viscus impairment are documented within the outline of Specific Product Characteristics (SPC). Paracetamol’s effectiveness proof, remarkably using for low back pain and degenerative arthritis, has been questioned [3]. Even so, on these precautions it’s enclosed as a first-line analgesic in tips for several forms of painful conditions, with a recommendation dose of 1g (maximum) fourfold on a daily basis [4,5]. To assessed effectiveness and safety of paracetamol we tend to aren’t cognizant of any clinical trials, specifically in older individuals. Patients aged over sixty five years might have enclosed in some clinical trials, most trials exclude frail older individuals with multimorbidity [6]. Paracetamol was first synthesized by Morse in 1878 and introduced for medical usage in 1883. However, because of delusion of its safety profile, it enjoyed solely restricted use till the fifties, once with chemicals similar, and up till then most well-liked analgesic, painkiller was withdrawn as a result of nephrotic toxicity. Currently in all probability, paracetamol is that the most typically used drug worldwide, out there over the counter, utilized in the majority ages, and forming step one of the UN agency analgesic ladder.
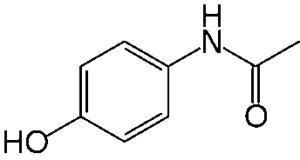
Figure 1: Chemical structure of paracetamol.
It is on the global World Health Organization’s (WHO) list of essential medicines, that lists the foremost effective and safe drug required in an exceedingly health system [7]. Paracetamol is obtainable as a generic name with trade names together with tylenol and anodyne, among others. The wholesale worth of paracetamol within the developing world is a smaller amount than US$ 0.01 per dose. Within the US, its prices regarding US$ 0.04 per dose. In 2016, it had been the seventeenth most prescribed medication within the US, with quite twenty-nine million prescriptions [8].
Mechanism of Action
It is stunning to grasp that after quite a century, the precise mechanism of action of paracetamol remains to be determined. There are some proof for variety of central mechanisms of paracetamol, as well as effects on prostaglandin (PG) production, and on opioid, gas oxide (NO), serotonergic, and cannabinoid pathways, with combination of interconnected pathways (Fig. 2).
Figure 2: The classic WHO analgesia ladder.
Paracetamol: A Prodrug of that AM404 is that Active Metabolite
Paracetamol (N-acetyl-para-aminophenol or Acetaminophen) metabolised and undergoes a deacetylation to p-aminophenol within the liver however conjointly within the central nervous system [9]. Following its viscus deacetylation to p-aminophenol, is metabolized within the brain by the carboxylic acid organic compound hydrolase (FAAH) to provide N-arachidonoylphenolamine (AM404) [3-5]. It has been prompt that AM404 is also liable for the analgesic mechanism of paracetamol [9,10].
The materia medica of AM404 formation by paracetamol within the central nervous system has been studied and justify by 2 totally different teams [9,11].
Högestätt and collaborators have shown that once twenty min administration of intraperitoneal injections of p-aminophenol (10, 30 and 100 mg/kg) or paracetamol (30, 100 and 300 mg/kg), these compounds were regenerate to AM404 at the doses of 3, 2, 44 and 667 pmol/g and 0.14, 1.6 and 10.3 pmol/g, severally [9]. Once administration of paracetamol in rats that is deuterium-labelled, they detected hydrogen atom labelled AM404 and p-aminophenol within the brain. They showed that, formation of p-aminophenol was gift altogether tissues, however highest levels within the liver area unit according which AM404 was primarily found within the brain. The later results were confirmed in an exceedingly recent study of Murasamatsu et al. (2016) [11].
Murasamatsu et al. (2016) incontestable the conversion of Tempra in AM404 in rats treated with orally paracetamol (20 mg/ kg), and therefore the peak of AM404 concentration obtained was 150 pg/g at the half-life worth of 0.3 h [11].
AM404 may be a potent agonist of the TRPV1 receptor that is transient receptor potential vanilloid kind one, associate degree Anandamide Membrane Transporter (AMT) blocker, a low-affinity substance of the sort one Cannabinoid receptor (CB1) and what is more, it has been shown that AM404 induces physiological condition and physiological condition in animal models [9,12-16].
Some studies have explained the impact of AM404 in modifying inflammation and aerophilous stress. Its effects on dipping aerophilous stress are related with the presence of a phenolic resin cluster in its structure [17].
Different Molecular Targets of AM404
- COX Enzyme: The first past hypothesis for the action of paracetamol, projected by Flower and Vane, was the inhibition of COX [18]. Paracetamol show solely weak inhibitory impact on autacoid production in cell culture, with IC50 values of 100μM [19]. In cell cultures, inhibition of COX enzyme by paracetamol was discovered in numerous kinds of cell, brain slices. On animal’s studies, paracetamol reduced autacoid in spinal fluid [20], in brain and within the medulla spinalis. Curiously, AM404 was shown to be AN matter of COX catalyst on isolated cyclooxygenase-1 and cyclooxygenase.
- TRPV1 Receptor: Recently studies have shown that AM404 is additionally a potent matter of the chemical irritant receptor named as TRPV1, as reported in patch-clamp experiments [21]. Stimulatingly, native injection of AM404 within the paw of mice resulted in pain behaviour (licking and lifting of the injected paw), a behaviour not found in TRPV1−/− mice [22].
The contribution of TRPV1 receptor to the action of paracetamol has been shown by each genetic and medical specialty approaches to inhibit it. Pharmacologically blockage of TRPV1 receptor by capsazepine in rats additionally minimise the analgesic impact of paracetamol [22].
- CB1 Receptor: AM404 is additionally capable to indirectly activation of the cannabinoid receptor CB1 by inhibiting the degradation and re-uptake of anandamide [23,24]. Study showing the involvement of this receptor within the action of paracetamol is CB1 knockout mice and rats pre-treated with a particular CB1 antagonist (AM251) were insensitive to paracetamol [22]. From these results, we have a tendency to show that the analgesic result of p-aminophenol was additionally suppressed or decrease by AM251 [25-27]. Remarkably, it absolutely was shown during a neuropathic rat pain model that the synergic or additive result of anti-nociception of paracetamol with gabapentin, tramadol and memantine was attenuated by pre-treatment with AM251 [28-30]. Within the same study, the intrinsic analgesic result of gabapentin, tramadol or memantine, wasn’t full of CB1 receptor antagonist.
- Serotoninergic Pathway Activation: Tjolsen et al., (1991) and Pini et al., (1996) show the involvement of the serotonergic system within the action of paracetamol [31,32]. They show that the analgesic impact of paracetamol was decrease when lesion of the serotonergic bulbospinal pathway by 5-6-dihydroxytryptamine or total reduction of the central monoamine neurotransmitter (5-HT) synthesis by p-chlorophenylalanine.
This results were proved by other team exploitation 5,7-dihydrixytryptamine [33,34]. Some study say that paracetamol failed to bind with 5-hydroxytryptamine receptors [35,36]. Prompted investigation of the mobilization of the monoamine neurotransmitter. The results showed that paracetamol exaggerated in a very dose-dependent manner the tissue concentrations of 5-HT within the cortex, neural structure, striatum, hippocampus and neural structure [35, 37].
This study showed that the spinal role of 5-HT1A, 5-HT2A, 5-HT2C and 5-HT7 receptor subtypes of monoamine neurotransmitter receptors was concerned within the action of paracetamol [33,38-44].
However, studying on the involvement of 5-HT3 receptors yielded conflicting results in each animals and humans [38,39,44-50].
New Methods to Alleviate Pain: Medical Specialty Vectorization to Focus on Brain TRPV1 Receptors
A high-concentration of chemical irritant, associate V-E Day patch (Qutenza®) is employed clinically for alleviate neuropathic pain in Europe and also the USA. It’s been recommended that it’s because of defunctionalisation of peripheral TRPV1 [51,52].
To validate this strategy, we have a tendency to study once more, with ED Högestätt (2005) and PM Zygmunt (2000), 4-Hydroxy3-Methoxybenzylamine (HMBA), a primary methane series analog of p-aminophenol [9,12]. HMBA created olvanil and arvanil in-vitro in brain homogenates and in-vivo in mouse brain [34].
Conclusion
In summary, we have a tendency to provide proof that show that paracetamol may be a prodrug that desires to overcome a two-step metabolism to create AM404, its active matter that mediates the analgesic impact via completely different supra-spinal targets to activate the bulbospinal serotonergic pathways. AM404 interferes in many steps of the assembly of prostaglandins in LPS-activated neuroglia. This study provide new important perceptions on the potential medicinal drug activity of AM404 and provides new mechanisms in respect of the central action of paracetamol within the modulation of autacoid production by neuroglia.
References
- Joint Formulary Committee. British National Formulary. Edition 75. London: BMJ Group and Pharmaceutical Press. 2018.
- Panadol original tablets. Summary of product characteristics, UK. GlaxoSmithKine Consumer Healthca (UK) Trading Limited, 2018.
- Moore A, Paracetamol: widely used and largely ineffective. Cochrane. 2018.
- National Institute of Health and Care Excellence. Clinical knowledge summaries: dysmenorrhea- primary dysmenorrhea. 2018.
- British Geriatric Society. Guidance on the management of pain in older people. 2013;42:1-57.
- MacLachlan AJ, Bath S, Naganathan V, Hilmer SN, Le Couter DG, Gibson SJ, et al. Clinical pharmacology of analgesic medicines in older people: impact of frailty and cognitive impairment. Br J Clin pharmacol. 2011;71:351-64.
- “WHO Model List of Essential Medicines (19th List)”. World Health Organization; 2016.
- https://clincalc.com/DrugStats/Top300Drugs.aspx. [Last accessed on 01 May 2020].
- Högestätt ED, Jönsson BA, Ermund A, Andersson DA, Björk H, Alexander JP, et al. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J Biol Chem. 2005;280(36):31405-12.
- Mallet C, Daulhac L, Bonnefont J, Ledent C, Etienne M, Chapuy E, et al. Endocannabinoid and serotonergic systems are needed for acetaminophen-induced analgesia. Pain. 2008;139(1):190-200.
- Muramatsu S, Shiraishi S, Miyano K, Sudo Y, Toda A, Mogi M, et al. Metabolism of AM04 from Acetaminophen at Human therapeutic dosages in the rat brain. Anesth Pain Med. 2016;17:1-5.
- Zygmunt PM, Chuang H, Movahed P, Julius D, Hogestatt ED. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur J Pharmacol. 2000;396: 39-42.
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094-7.
- Ottani A, Leone S, Sandrini M, Ferrari A, Bertolini A. The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors. Eur J Pharmacol. 2006; 531:280-1.
- Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A. Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proc Natl Acad Sci USA. 2004;101:8756-61.
- Mitchell VA, Greenwood R, Jayamanne A, Vaughan CW. Actions of the endocannabinoid transport inhibitor AM404 in neuropathic and inflammatory pain models. Clin Exp Pharmacol Physiol. 2007;34:1186-90.
- Marsicano G, Moosmann B, Hermann H, Lutz B, Behl C. Neuroprotective properties of cannabinoids against oxidative stress: role of the cannabinoid receptor CB1. J Neurochem. 2002;80:448-56.
- RJ Flower, JR Ane. Inhibition of prostaglandin synthetase in brain explains the antipyretic activity of paracetamol (4-acetamidophenol). Nature. 1972;240:410-11.
- GG Graham, RO Day, MK Milligan, JB Ziegler, AJ Kettle. Current concepts of the actions of paracetamol (acetaminophen) and NSAIDs. Inflammo Pharmacol. 1999;7:255-63.
- W Feldberg, KP Gupta, Pyrogen fever and prostaglandin-like activity in cerebrospinal fluid. J Physiol. 1973;228(1973):41-53.
- N Kerckhove, C Mallet, A François, M Boudes, J Chemin, T Voets, et al. Ca (v) 3.2 calcium channels: the key protagonist in the supraspinal effect of paracetamol. Pain. 2014;155:764-72.
- C Mallet, DA Barriere, A Ermund, BA Jonsson, A Eschalier, PM Zygmunt, et al. TRPV1 in brain is involved in acetaminophen-induced antinociception. PLoS One. 2010;5:1-11.
- ST Glaser, NA Abumrad, F Fatade, M Kaczocha, KM Studholme, DG Deutsch. Evidence against the presence of an anandamide transporter. Proc Natl Acad Sci. 2003;100:4269-74.
- M Beltramo, N Stella, A Calignano, SY Lin, A Makriyannis, D Piomelli. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094-7.
- AT Hama, J Sagen. Cannabinoid receptor-mediated antinociception with acetaminophen drug combinations in rats with neuropathic spinal cord injury pain. Neuropharmacol. 2010;58:758-66.
- A Tjolsen, A Lund, K Hole. Antinociceptive effect of paracetamol in rats is partly dependent on spinal serotonergic systems. Eur J Pharmacol. 1991;193:193-201.
- LA Pini, M Sandrini, G Vitale. The antinociceptive action of paracetamol is associated with changes in the serotonergic system in the rat brain. Eur J Pharmacol. 1996;308:31-40.
- A Dogrul, M Seyrek, EO Akgul, T Cayci, S Kahraman, H Bolay. Systemic paracetamol-induced analgesic and antihyperalgesic effects through activation of descending serotonergic pathways involving spinal 5-HT (7) receptors. Eur J Pharmacol. 2011;8:1-11.
- DA Barriere, C Mallet, A Blomgren, C Simonsen, L Daulhac, F Libert, et al. Fatty acid amide hydrolase dependent generation of antinociceptive drug metabolites acting on TRPV1 in the brain. PLoS One. 2013;8:1-13.
- LA Pini, M Sandrini, G Vitale. The antinociceptive action of paracetamol is associated with changes in the serotonergic system in the rat brain. Eur J Pharmacol. 1996;308:31-40.
- T Pelissier, A Alloui, F Caussade, C Dubray, A Cloarec, J Lavarenne, et al. Paracetamol exerts a spinal antinociceptive effect involving an indirect interaction with 5-hydroxytryptamine 3 receptors: in-vivo and in-vitro evidence. J Pharmacol Exp Ther. 1996;278:8-14.
- JP Courade, F Caussade, K Martin, D Besse, C Delchambre, N Hanoun, et al. Effects of acetaminophen on monoaminergic systems in the rat central nervous system. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:534-7.
- A Alloui, T Pelissier, C Dubray, J Lavarenne, A Eschalier. Tropisetron inhibits the antinociceptive effect of intrathecally administered paracetamol and serotonin. Fundam Clin Pharmacol. 1996;10:406-7.
- A Alloui, C Chassaing, J Schmidt, D Ardid, C Dubray, A Cloarec, et al. Paracetamol exerts a spinal, tropisetron-reversible, antinociceptive effect in an inflammatory pain model in rats. Eur J Pharmacol. 2002;443:71-7.
- J Bonnefont, A Alloui, E Chapuy, E Clottes, A Eschalier. Orally administered paracetamol does not act locally in the rat formalin test: evidence for a supraspinal, serotonin-dependent antinociceptive mechanism. Anesthesiol. 2003;99:976-81.
- J Bonnefont, E Chapuy, E Clottes, A Alloui, A Eschalier. Spinal 5-HT1A receptors differentially influence nociceptive processing according to the nature of the noxious stimulus in rats: effect of WAY-100635 on the antinociceptive activities of paracetamol, venlafaxine and 5-HT. Pain. 2005;114:482-90.
- J Bonnefont, L Daulhac, M Etienne, E Chapuy, C Mallet, L Ouchchane, et al. Acetaminophen recruits spinal p42/p44 MAPKs and GH/IGF-1 receptors to produce analgesia via the serotonergic system. Mol Pharmacol. 2007;71:407-15.
- JP Courade, C Chassaing, L Bardin, A Alloui, A Eschalier. 5-HT receptor subtypes involved in the spinal antinociceptive effect of acetaminophen in rats, Eur J Pharmacol. 2001;432:1-7.
- P Girard, Y Pansart, MC Coppe, B Niedergang, JM Gillardin. Modulation of paracetamol and nefopam antinociception by serotonin 5-HT (3) receptor antagonists in mice. Pharmacol. 2009;83:243-46.
- J Liu, AR Reid, J Sawynok. Antinociception by systemically-administered acetaminophen (paracetamol) involves spinal serotonin 5-HT7 and adenosine A1 receptors, as well as peripheral adenosine A1 receptors. Neurosci Lett. 2013;536:64-8.
- F Libert, J Bonnefont, E Bourinet, E Doucet, A Alloui, M Hamon, et al. Acetaminophen: a central analgesic drug that involves a spinal tropisetronsensitive, non-5-HT (3) receptor-mediated effect. Mol Pharmacol. 2004;64:728-34.
- V Minville, O Fourcade, JX Mazoit, JP Girolami, I Tac. Ondansetron does not block paracetamol-induced analgesia in a mouse model of fracture pain. Br J Anaesth. 2011;206:112-8.
- R Jokela, J Ahonen, E Seitsonen, P Marjakangas, K Korttila. The influence of ondansetron on the analgesic effect of acetaminophen after laparoscopic hysterectomy. Clin Pharmacol Ther. 2010;87:672-8.
- G Pickering, MA Loriot, F Libert, A Eschalier, P Beaune, C Dubray. Analgesic effect of acetaminophen in humans: first evidence of a central serotonergic mechanism. Clin Pharmacol Ther. 2006;79:371-8.
- L Ramirez, J Cros, B Marin, P Boulogne, A Bergeron, GE de Lafont, et al. Analgesic interaction between ondansetron and acetaminophen after tonsillectomy in children: The Paratron randomized controlled trial. Eur J Pain. 2015;19:661-8.
- E Tiippana, K Hamunen, V Kontinen, E Kalso. The effect of paracetamol and tropisetron on pain: experimental studies and a review of published data. Basic Clin Pharmacol Toxicol. 2013;112:124-31.
- P Anand, K Bley. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011;107:490-502.
- www.researchgate.net/figure/Proposed-sequential-mechanisms-for-the-antinociceptive-effect-of-paracetamol-1_fig3_317152037 [Last accessed on 01 May 2020].
- https://en.wikipedia.org/wiki/Paracetamol [Last accessed on 01 May 2020].
- https://i1.wp.com/emcrit.org/wp-content/uploads/2017/06/ladder2.png [Last accessed on 01 May 2020].
- https://chiro.org/LINKS/FULL/NSAIDs_and_Musculoskeletal_Treatment.html [Last accessed on 01 May 2020].
- https://www.researchgate.net/figure/Proposed-sequential-mechanisms-for-the-antinociceptive-effect-of-paracetamol-1_fig3_317152037 [Last accessed on 01 May 2020].
This work is licensed under Attribution-NonCommercial-NoDerivs 2.0 Generic (CC BY-NC-ND 2.0) International License. With this license readers are free to share, copy and redistribute the material in any medium or format as long as the original source is properly cited.

