Neo-adjuvant PIPAC and Systemic Chemotherapy in Management of Synchronous Peritoneal Metastasis From Gastric Cancer
Bohlooli M1, Farmakis D2, Saroyan H2, Kazemi V1, Noskova I2, Omid R1, Spiliotis J2*
1Department of Surgery and Medical Oncology JAM Hospital, Tehran, Iran
2Department of Surgery, Surgical Oncology European Interbalkan Medical Center, Thessaloniki, Greece
*Correspondence author: John Spiliotis, MD, PhD, FASPSM, Department of Surgery, Surgical Oncology European Interbalkan Medical Center, Thessaloniki, Greece; Email: jspil@hotmail.gr
Citation: Spiliotis J, et al. Neo-adjuvant PIPAC and Systemic Chemotherapy in Management of Synchronous Peritoneal Metastasis From Gastric Cancer. Jour Clin Med Res. 2023;4(3):1-9.
Copyright© 2023 by Spiliotis J, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
| Received 11 Oct, 2023 | Accepted 26 Oct, 2023 | Published 04 Nov, 2023 |
Abstract
Introduction: Polycystic Ovarian Disorder (PCOD) is a prevalent endocrine illness in women of reproductive age. It has hormonal abnormalities, irregular menstrual cycles and tiny ovarian cysts. Lifestyle and food affect PCOD development and maintenance, coupled with Peritoneal metastasis from gastric cancer remains a major problem.
The aim of our study is a retrospective analysis in two different groups with used PIPAC as neo-adjuvant management of synchronous peritoneal metastasis.
Group A: 42 patients received as neo-adjuvant treatment 2 cycles of PIPAC (Doxorubicin 3 mgr/m2, cisplatin 10 mgr/m2) and 6 cycles of neo-adjuvant systemic chemotherapy with FLOT and we performed CRS+HIPEC (with cisplatin 80 mgr/m2, mitomycin 35 mgr/m2) for 90 min and 6 cycles of adjuvant systemic chemotherapy with FLOT.
Group B: 26 patients received 4 cycles of neo-adjuvant systemic chemotherapy with FLOT, we performed CRS+HIPEC as Group A and 4 cycles of systemic chemotherapy with FLOT.
For patients of Group A, the Median survival time is 46months.Group B patients had a median OS of 36 months.
For patients of Group A, the Median Disease Time (DFS) was 37 months. Group B patients had a median DFS of 30 months. For each degree increase in the PCI pre score and for the same treatment group, the PCI posr score increases by 0.573 degrees. Group 2, for the same PCIpre score, have a 163% higher PCI posr score. On patients with singet ring cell adenocarcinoma there is a statistical significance difference in OS for PIPAC group. Patients of group A had a median OS of 26 months. For patients of group B, the median OS was 14 months. Morbidity and mortality in both groups are 53,4% and 4,7% for Group A and 44,5% and 5,5% for Group B respectively. The neo-adjuvant PIPAC with systemic chemotherapy might be a promising approach for patients with peritoneal metastasis from gastric cancer. PIPAC is a safe and well tolerated procedure.
Keywords: Gastric Cancer; Peritoneal Metastasis; Cytoreductive Surgery; Hyperthermic Intraperitoneal Chemotherapy
Article Type
Research Article
Introduction
Gastric Cancer (GC) remains the fourth most common cancer and the second leading cause of cancer-related deaths worldwide, although its incidence and mortality have decreased over the last decades [1]. Generally, most GC patients are diagnosed as advanced stage except countries with national screening programs such as South Korea and Japan, because early-stage GC is commonly asymptomatic [2]. Therefore, the prognosis of patients with GC remains poor and the 5-year overall survival rate for all the patients diagnosed with GC is only 40-60% in Asia and 24.5% in Europe [3,4]. Among advanced GC cases, peritoneal implantation is one of the most debilitating and most common forms of metastasis. It was reported that peritoneal dissemination rate of GC patients was about 14% at initial examination and the median survival time was 3-6 months [5]. Peritoneal Metastasis (PM) of GC was regarded as a terminal disease until the early 1990s because it was considered as unresectable and response from systemic chemotherapy was very limited [6]. Peritoneal Metastasis (PM) in any solid cancer represents a disseminated disease and is associated with a dismal prognosis. Various guidelines recommend systemic chemotherapy as a therapeutic option for PM in a well-preserved patient and BSC is the norm in a terminally ill patient [7]. Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) has generated a considerable interest in the last three decades in the management of PM. Various studies have suggested that CRS/HIPEC may even prove to be a curative treatment in a select group of patients who have isolated low volume peritoneal disease [8]. A retrospective French study of 277 patients with GC related PM indicated a significant survival benefit with CRS/HIPEC compared to CRS alone (median survival 18.8 vs 12.1 months) [9]. A recent prospective study of 35 patients with GC related PM (PCI < 6) also highlighted a notable median survival of 19 months when they were treated with CRS/HIPEC [10].
The strict criteria for selecting the patients for CRS/HIPEC is warranted in order to avoid the associated postoperative morbidity and mortality in those patients in whom this procedure will be futile oncologically and will not add to either progression free survival (PFS) or OS [11]. However, these strict criteria leave a large room for a significant number of patients who are not fit for CRS/HIPEC in view of high-volume peritoneal disease where CRS/HIPEC is likely to leave a significant gross residual disease.
Pressurized Intra Peritoneal Aerosol Chemotherapy (PIPAC), a recently described new surgical technique to administer chemotherapy directly to the peritoneum under pressure, has added a new dimension to the armamentarium of the oncologists to address the PM in those patients who are not suitable candidates for CRS/HIPEC [12]. The first report of successful application of PIPAC in three patients with PM, including one with gastric cancer, was published in 2014 [13]. Since then, a few articles have described the effectiveness and the safety of PIPAC in PM in patients with cancers of various origins. In our study we present a retrospective analysis of two groups with different protocols of neo-adjuvant management of synchronous peritoneal metastasis from gastric cancer. One with neo-adjuvant systemic chemotherapy plus Pressurized Intraperitoneal Aerosol chemotherapy and the other only neo-adjuvant systemic chemotherapy.
Patients and Methods
From 2016, patients with synchronous PM from Gastric cancer were studied retrospectively with prospective protocols from two different centers and divided into the following groups. Group A: 60 patients include in Group A and 18 excluded from the study due to progress disease or toxicity from chemotherapy. 42 remaining patients with PM from gastric cancer (28 male and 14 female), which received two cycles of neo-adjuvant PIPAC with Doxorubicin 3 mgr/m2 and Cisplatin 10 mgr/m2 and then 6 cycles of neo-adjuvant systemic chemotherapy with FLOT as presented in (Fig. 1) and then we performed CRS plus HIPEC with Cisplatin 80 mgr/m2 and Mitomycin 35 mgr/m2 for 90 minutes and then 6 cycles of systemic chemotherapy with FLOT, patients’ characteristics are presented in Table 1.
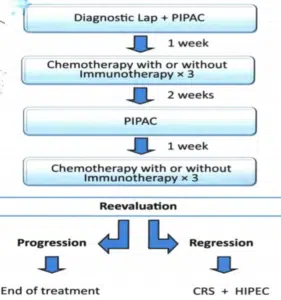
Figure 1: Cycles of neo-adjuvant systemic chemotherapy with FLOT.
№ | Age | Sex | Loc | PCI1 | PCI2 | PC3 | Hist | CC | LN | Compl. |
1 | 46 | ♂ | C | 13 | 10 | 7 | As | 0 | 8/22 | – |
2 | 53 | ♂ | B | 12 | 8 | 5 | A | 0 | 6/28 | 2 |
3 | 58 | ♀ | B | 9 | 6 | 4 | A | 0 | 4/26 | 1 |
4 | 36 | ♂ | C | 4 | 2 | 1 | As | 0 | 7/27 | 1 |
5 | 43 | ♂ | A | 6 | 3 | 2 | A | 0 | 3/29 | 1 |
6 | 63 | ♂ | C | 3 | 0 | 0 | A | 0 | 2/23 | 2 |
7 | 49 | ♀ | C | 6 | 4 | 1 | A | 0 | 4/36 | 2 |
8 | 56 | ♂ | B | 8 | 4 | 3 | A | 0 | 0/33 | – |
9 | 39 | ♂ | B | 6 | 3 | 1 | As | 1 | 7/28 | 1 |
10 | 70 | ♂ | A | 4 | 4 | 1 | A | 0 | 6/35 | 4 Post operdeath |
11 | 37 | ♀ | B | 12 | 6 | 3 | A | 1 | 12/31 | – |
12 | 58 | ♀ | C | 7 | 3 | 3 | A | 0 | 3/25 | – |
13 | 59 | ♂ | A | 5 | 3 | 2 | A | 0 | 1/18 | – |
14 | 64 | ♂ | B | 11 | 4 | 4 | A | 0 | 0/38 | 1 |
15 | 51 | ♀ | B | 6 | 4 | 0 | A | 0 | 2/44 | 1 |
16 | 62 | ♂ | C | 6 | 4 | 1 | As | 0 | 3/29 | 1 |
17 | 32 | ♂ | C | 10 | 4 | 3 | A | 0 | 0/29 | – |
18 | 73 | ♀ | B | 5 | 3 | 2 | A | 0 | 6/37 | – |
19 | 46 | ♂ | B | 8 | 6 | 3 | A | 0 | 1/25 | – |
20 | 73 | ♂ | C | 10 | 4 | 3 | As | 0 | 3/29 | 2 |
21 | 61 | ♀ | B | 7 | 4 | 4 | A | 0 | 2/31 | – |
22 | 68 | ♀ | B | 13 | 6 | 4 | As | 1 | 5/33 | – |
23 | 58 | ♂ | A | 3 | 2 | 2 | A | 1 | 7/27 | – |
24 | 55 | ♀ | A | 6 | 4 | 2 | A | 0 | 5/36 | 2 |
25 | 48 | ♀ | F | 5 | 3 | 2 | A | 0 | 0/25 | – |
26 | 63 | ♂ | B | 7 | 4 | 2 | A | 0 | 1/26 | 3 |
27 | 67 | ♂ | C | 4 | 4 | 0 | A | 0 | 2/19 | – |
28 | 62 | ♂ | C | 12 | 6 | 3 | As | 1 | 6/36 | – |
29 | 45 | ♂ | A | 4 | 0 | 0 | A | 0 | 4/29 | 3 |
30 | 71 | ♀ | B | 6 | 4 | 1 | A | 0 | 0/33 | 2 |
31 | 69 | ♂ | B | 10 | 6 | 1 | A | 0 | 0/28 | – |
32 | 62 | ♂ | B | 5 | 2 | 0 | A | 0 | 1/23 | 2 |
33 | 60 | ♂ | C | 6 | 4 | 1 | A | 0 | 2/32 | 4 Post operdeath |
34 | 54 | ♀ | A | 6 | 3 | 1 | A | 0 | 4/36 | – |
35 | 49 | ♂ | B | 8 | 4 | 2 | A | 1 | 2/18 | – |
36 | 53 | ♂ | C | 8 | 6 | 4 | As | 0 | 3/22 | – |
37 | 34 | ♂ | B | 6 | 5 | 2 | A | 0 | 2/25 | 2 |
38 | 65 | ♀ | B | 9 | 5 | 3 | A | 0 | 7/23 | 2 |
39 | 52 | ♀ | B | 8 | 6 | 4 | As | 1 | 3/29 | – |
40 | 66 | ♂ | A | 9 | 6 | 2 | A | 0 | 0/32 | 2 |
41 | 73 | ♂ | C | 4 | 3 | 0 | A | 0 | 1/30 | – |
42 | 62 | ♂ | B | 5 | 2 | 0 | A | 0 | 1/36 | 3 |
PCI1: Peritoneal Cancer Index in First Pipac; PCΙ2: Second Pipac; PC3: During CRS; CC: Completeness of Cytoreduction; LN: Lymph Nodes (+/total); A: Adenocarcinoma; AS: Adenocarcinoma Signet Ring morphology | ||||||||||
Table 1: Group A: PIPAC + 2 cycles neo ad. + Pipac + 2 cycles Neo à CRS + HIPEC à 4 cycles Chemotherapy.
Group B:
Group B: 26 patients include in Group B and from them 8 excluded from the study due to progression of the disease and toxicity of chemotherapy. The remain 18 patients with PM from gastric cancer include in the study 11 male and 7 female which received six cycles of neo-adjuvant systemic chemotherapy and then we performed CRS plus HIPEC as group A and then 6 cycles of systemic chemotherapy with FLOT (Table 2). All the details concerning the two groups’ patients’ characteristics are presented in Table 1 and 2, for both groups.
№ | PCI1 | PC2 | Hist | CC | LN | Compl. |
1 | 14 | 9 | A | 0 | 4/23 | 1 |
2 | 13 | 10 | As | 1 | 10/26 | 2 |
3 | 11 | 11 | As | 1 | 14/22 | 1 |
4 | 5 | 4 | A | 0 | 8/24 | 0 |
5 | 14 | 9 | A | 0 | 4/16 | 0 |
6 | 6 | 4 | As | 0 | 7/23 | 0 |
7 | 10 | 7 | A | 0 | 12/18 | 0 |
8 | 7 | 4 | A | 0 | 12/30 | 2 |
9 | 8 | 6 | A | 0 | 8/12 | 3 |
10 | 12 | 7 | A | 0 | 7/25 | 1 |
11 | 10 | 6 | A | 0 | 6/32 | 0 |
12 | 7 | 2 | A | 0 | 4/30 | 0 |
13 | 5 | 2 | A | 0 | 0/12 | 0 |
14 | 11 | 8 | As | 1 | 18/21 | 0 |
15 | 6 | 4 | A | 0 | 6/14 | 1 |
16 | 7 | 4 | A | 0 | 7/21 | 0 |
17 | 5 | 2 | A | 0 | 6/30 | 0 |
18 | 10 | 6 | As | 0 | 14/31 | 4 Post operdeath |
PCI1: Initial Diagnose; PC2: During Operation | ||||||
Table 2: Group B: 4 cycles Neo Adj. à CRS + HIPEC à 4 cycles Adj.
Morbidity and mortality are presented with Clavian -Dindo classification as described [14]. All data were analyzed using SPSS software version 25.0 (SPSS Inc., Chicago, IL) for Windows and p- values<0.05 were statically significant. Continuous data are reported as mean (standard deviation) and categorical data are reported as number (percentage). Time-to-event outcomes were estimated using Kaplan-Meier curves. The log-rank test was used to assess the effect of the treatment regimen (group A vs group B) on Overall Survival (OS) and Disease-Free Survival (DFS). A multiple linear regression model was established to assess the association between PCI post-test score and: a) PCI pre-test score, b) treatment regimen.
Results
Of the 60 patients included in the analysis the mean age was 55 years, with standard deviation ±10.9. Most patients were male (65 %). The mean score of preoperative PCI was 7.6 (SD=3) and 3.2 (SD=2.6) of postoperative PCI (Table 3). The mean length of follow-up was 20.7±10.7 months and the median follow-up duration was 19 months ranging from 0 to 48 months. The mean time after treatment during which no sign of cancer is found was 12.9±10.2 months and the median time was 11 months ranging from 0 to 33 months. Sixteen patients (26,7%) have died by the end of follow-up whereas 44 (73.3%) were the censored cases (Table 4).
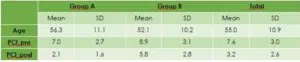
Table 3: Mean score of preoperative PCI.
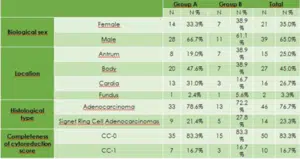
Table 4: Postoperative PCI.
The Morbidity and Mortality
Complication and mortality rates are not statistically significant between the two groups. The presentence of patients’ exclusion due to progress of the disease or due to chemotherapy toxicity were 18/60 (30%) in Group A and 8/26 (30,7%) in Group B similar in both groups.
Group A: morbidity 52,4% and mortality 4,7%
Group B: morbidity 44,5% and mortality 5,5%
Disease-Free Survival Pet Treatment Regimen Group
Group B patients had a median DFS of 30 months. For patients of Group A, the median survival time was 37 months. The survival distributions for the treatment regimen group were not statistically significantly different, x2(1) =0.177, p=0.278.
Multiple Linear Regressions
A multiple regression was run to predict PCI post score from treatment regimen group and PCI pre score. There was linearity as assessed by partial regression plots and a plot of studentized residuals against the predicted values. There was independence of residuals, as assessed by a Durbin-Watson (statistic of 2.077). There was homoscedasticity, as assessed by visual inspection of a plot of studentized residuals versus unstandardized predicted values. There was no evidence of multicollinearity, as assessed by tolerance values greater than 0.1 (0.912 for treatment regimen group and 0.912 for PCI pre score). There were no studentized deleted residuals greater than ± 3 standard deviations, no leverage values greater than 0.2 and values for Cook’s distance above 1. The assumption of normality was met, as assessed by a Q-Q Plot. The multiple regression model statistically significantly predicted PCI_post score, F (2,57) =116.131, p<0.001, adj. R2=0.796. Both variables added significantly to the prediction, p<0.001.
The prediction equation was:
PCI_post = -4.544 + 2.628* Group + 0.573* PCI_pre (Table 4)
For each degree increase in the PCI_pre score and for the same treatment group, the PCI_post score increases by 0.573 degrees. Group 2, for the same PCI_pre score, have a 163% higher PCI_post score.
That means better response in Group A, so the PIPAC regimen improved the Completeness of Cytoreduction in patients of Group A (Fig. 2-4). The survival distributions between the tumor locations in both groups were not statistically significantly different P≤0.152. The same results about the four different locations (antrum, body, cardia and fundus) were not significantly different between the two groups. On patients with signet ring cell adenocarcinoma there is a statistical significance difference in OS for PIPAC group. Patients of group A had a median OS of 26 months (95% CI: 16.4-35.6). For patients of group B, the median OS was 14 months (95% CI: 10.5-17.5). A log rank test was conducted to determine if there were differences in the survival distribution for the treatment regimen group. The survival distributions for the treatment regimen group were statistically significantly different, χ2(1) = 5.775, p = 0.016.
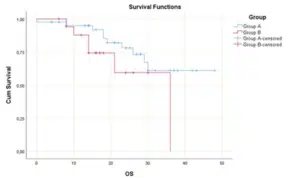
Figure 2: Survival functions in patients of all groups.
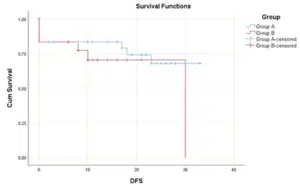
Figure 3: Survival functions with Median Disease Time in patients of all groups.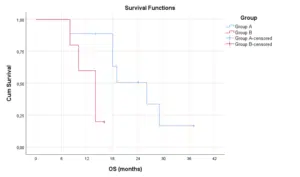
Figure 4: Comparison of survival functions in patients of all groups.
Discussion
Gastric Cancer is associated with poor prognosis with a 5-year overall survival is around 17-23% depending on the tumor stage and histology. Especially, signet-ring histology is associated with dismal prognosis. The treatment is surgery in the early stages and neo-adjuvant chemotherapy in more advanced stage in order to down staging the disease, following a curative (Ro) resection. The peritoneal Metastasis (PM) was reported in 25% during initial diagnosis with worse prognosis of 6-10 months with conventional treatment [7]. One of the most important therapeutic roads during the last 2 decades for PM is to combine systemic chemotherapy and logo regional treatment [14]. In well selected patients with PM from gastric cancer the perioperative chemotherapy with Cytoreductive Surgery and Hyper Thermic Intraperitoneal Chemotherapy (HIPEC) has been shown improved over all survival with a care rate, especially in low volume peritoneal deposits of 11% [15,16]. The last 10 years Pressurized Intraperitoneal Chemotherapy (PIPAC) as an alternative approach to treat PM for patients who are not eligible for CRS and HIPEC are onset [17].
In our study we compare the combination of PIPAC not as a novel palliative approach but as neo-adjuvant treatment together with systemic neo-adjuvant chemotherapy aiming to down staging synchronous peritoneal metastasis from gastric cancer and to improve the survival in these patients. In the present trial the PIPAC group demonstrates a significant down staging in peritoneal deposits as expressed by Peritoneal Cancer Index (PCI) in the start of therapy versus the conventional treatment with neo adjuvant systemic chemotherapy only. The treatment was well tolerated without any difference in morbidity and mortality between the two groups. At first PIPAC the PCI in group A was relatively high and progressively until laparotomy after two cycles of neo-adjuvant PIPAC the PCI decreased. This management of neo-adjuvant systemic and neo-adjuvant PIPAC decreases PCI statistically significantly better than group B with neo-adjuvant chemotherapy alone (2.1±1.6 vs 5.8±2.8 p<0.002) and offers a better approach for completeness of cytoreduction. This data should be considered preliminary and interpreted with caution.
First, as a priority in our institutions is to perform CRS and HIPEC in all eligible patients, rather than PIPAC, but PIPAC can be proposed to patients who are not eligible for CRS and HIPEC. Second, it is feasible to use PIPAC, a neo-adjuvant tool together with systemic chemotherapy in order to achieve more CCo resections and our study demonstrates this benefit. The probability of overall survival and disease-free survival was higher in the gastric cancer patients of Group A. In fact, the difference between both survival curves (Group A vs Group B) did not achieved significance, although the number of patients is limited but this suggest that neo-adjuvant PIPAC might have a positive impact on survival and demands prospective randomized trials. Another important question which arises is the number of neo-adjuvants PIPAC cycles need to decrease. PCI and transform the diffuse peritoneal disease into localized disease or which is the ideal time interval for PIPAC procedure [18,19].
Conclusion
In conclusion we propose that neo-adjuvant PIPAC with systemic chemotherapy might be a promising approach for patients with peritoneal metastasis from gastric cancer. PIPAC is a simple, safe and well + tolerated procedure. We need methodological preconditions (time, interval, drugs) to design a clinical trial to re-evaluate the potential role of PIPAC as neo-adjuvant therapy in gastric cancer patients with synchronous peritoneal metastatic.
Author Contributions
All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
- Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric adenocarcinoma. Nat Rev Dis Primers. 2017;3:17036.
- Lu M, Yang Z, Feng Q, Yu M, Zhang Y, Mao C, et al. The characteristics and prognostic value of signet ring cell histology in gastric cancer: A retrospective cohort study of 2199 consecutive patients. Medicine (Baltimore). 2016;95(27):e4052.
- Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. 2019;393(10184):1948-57.
- Wagner AD, Nicholas LX Syn, Moehler M, Grothe W, Yong WP, Tai BC, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;2017(8).
- Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26-38.
- Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric cancer, version 3. NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw JNCCN. 2016;14:1286-312.
- Morales-Soriano R, Esteve-Pérez N, Segura-Sampedro JJ, Cascales-Campos P, Barrios P. Spanish Group of Peritoneal Malignancy Surface (GECOP). Current practice in cytoreductive surgery and HIPEC for metastatic peritoneal disease: Spanish multicentric survey. Eur J Surg Oncol. 2018;44:228-36.
- Bonnot PE, Piessen G, Pocard M, Meunier B, Bereder JM, Abboud K, et al. CYTO-CHIP: cytoreductive surgery versus cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis: a propensity-score analysis from BIG RENAPE and FREGAT working groups. J Clin Oncol. 2018;36:8.
- Rihuete Caro C, Manzanedo I, Pereira F, Carrion-Alvarez L, Serrano Á, Pérez-Viejo E. Cytoreductive surgery combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in patients with gastric cancer and peritoneal carcinomatosis. Eur J Surg Oncol. 2018;44:1805-10.
- Ji Z-H, Peng K-W, Yu Y, Li X-B, Yonemura Y, Liu Y, et al. Current status and future prospects of clinical trials on CRS + HIPEC for gastric cancer peritoneal metastases. Int J Hyperth. 2017;33:562-70.
- Garg PK, Brandl A, Rau B. Hyperthermic intraperitoneal chemotherapy – fading perspective in the light of modern systemic chemotherapy? Visc Med. 2018;34:412-6.
- Alberto M, Brandl A, Garg PK, Gül-Klein S, Dahlmann M, Stein U, et al. Pressurized intraperitoneal aerosol chemotherapy and its effect on gastric-cancer-derived peritoneal metastases: an overview. Clin Exp Metastasis. 2019.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications a new proposal with evaluation in a cohort of 6336 patients and results of a surgery. Ann. Surg. 2004;240(2):205-13.
- Yap Yi DR, Wong Min JS, Tan XQ, Tan Joey WS, Chia CS, Ong Johnny CA. Effect of HIPEC on Peritoneal recurrence in Peritoneal metastasis treated With Cytoreductive Surgery: A systematic Review. Frontiers in Oncol. 2021.
- Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Annals Surgical Oncology. 2011;18:1575-81.
- Girshally R, Demotroder C, Albayrak N, Zieren J, Tempfer C, Reymond MA. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surgical Oncol. 2016;14:253.
- Struller F, Horvath PH, Solass W, Weinreich FJ, Strumberg D, Kokkalis MK. Pressurized intraperitoneal aerosol chemotherapy with low-dose cisplatin and doxorubicin (PIPAC C/D) in patients with gastric cancer and peritoneal metastasis: a phase II study. Ther Adv Med Oncol. 2019;11.
- Garg PK, Jara M, Alberto M, Rau B. The role of pressurized intraperitoneal aerosol chemotherapy in the management of gastric cancer: A systemic review. Pleura and Peritoneum. 2019;20180127.
This work is licensed under a Creative Commons Attribution 2.0 International License.


