Yuko Watanabe1, Kenshi Yamasaki2,3*
1Watanabe Dermatology and Plastic Surgery Clinic, Matsuyama, Ehime, Japan
2Tohoku University School of Medicine, Sendai, Miyagi, Japan
3Aloop Clinic and Lab, Ginza, Tokyo, Japan
*Correspondence author: Kenshi Yamasaki, MD, Ph.D., Tohoku University School of Medicine, Sendai, Miyagi, Japan and Aloop Clinic and Lab, Ginza, Tokyo, Japan; Email: [email protected]
Published Date: 17-02-2024
Copyright© 2024 by Yamasaki K, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Effective treatments for pediatric periorificial dermatitis are limited. We assessed the clinical utility of oral Clarithromycin (CLR) in pediatric patients with periorificial dermatitis.
Methods and Findings: A retrospective medical record review of pediatric patients with periorificial dermatitis was performed. A total of 39 pediatric patients with periorificial dermatitis received prescription of oral CLR during January 2021 and February 2023. The median age at diagnosis was 5.3 ± 3.9 years (interquartile rage 2-9). Except 8 who did not revisit the clinic and could not obtain safety data, none of 31 experienced adverse events during CLR meditation. Among 31 cases who revisited the clinic, we selected 25 cases for efficacy evaluation and excluded 6 cases who stopped medication by their own reason and/or did not take medication regularly as prescribed. Twenty-five cases included 13 females and 12 meles and average age was 6.2 ± 4.2 years (interquartile rage 2-9). Twenty-three cases achieved Complete Response (CR) by CLR: 18 achieved CR in 4 weeks and 5 achieved CR in 8 weeks. One case showed partial response (PR) by CLR and one case worsen after CLR administration with cessation of TCI. Among 23 CR cases, 7 cases (30%) had relapsed during 1.5 to 19 months after CR. All of relapse cases achieved CR by readministration of CLR.
Conclusion: Oral clarithromycin is an effective and well tolerated therapeutic option for pediatric patients with periorificial dermatitis.
Keywords: Perioral Dermatitis; Periorificial Dermatitis; Pediatric Dermatology; Clarithromycin; Topical Calcineurin Inhibitor; Topical Glucocorticoid
Abbreviations
POD: Perioral Dermatitis; CLR: Clarithromycin; TGC: Topical Glucocorticoids; TCI: Topical Calcineurin Inhibitor; CR: Complete Response; PR: Partial Response; IQR: Interquartile Range
Introduction
Perioral Dermatitis (POD) is characterized by red to orange-colored papules and pustules affecting the skin of perioral region, typically on mouth and nasolabial fold area. A lesion appears as papules and turns to erythema with scaly appearance. Lesions are persistent and repeatedly appeared. POD involves cutaneous lip but not mucosal region including vermilion lip. Periorbital dermatitis refers papules appeared on eye lids. Because POD and Periorbital dermatitis often appear simultaneously and show similar papular lesions, a term of periorificial dermatitis is proposed to include concept of both POD and Periorbital dermatitis.
Etiology of POD is not fully elucidated. Sensitivity to the sun light, seborrhea, topical fluorinated corticosteroid, fluorinated toothpaste, hormonal factors in female and pregnancy, candida albicans, fusobacterium and others have been discussed as causes or exacerbating factors of POD [1,2]. However, none of them cannot universally explain the cause of POD. Because external environmental factors have been discussed for pathogenesis of POD and because symptoms of POD have similarity with rosacea, innate immunity-mediated host responses to environmental conditions might be involved in POD pathogenesis like the pathogenesis of rosacea [3-5].
POD is often observed in young adults and children and treatment of pediatric POD is challenging. No medication is officially approved for both adults and pediatric POD. Although tetracyclines are frequently used for treatment of adult POD, tetracyclines have serious adverse effects for children, such as permanent tooth staining. Because of pathological similarity to rosacea, topical metronidazole is used for both adult and pediatric POD [6-8]. It is reported that Topical Calcineurin Inhibitors (TCIs) show favorable outcome for pediatric cases [9]. Combination of TCIs and topical/oral metronidazole are also used for pediatric POD [10]. However, pediatric POD often failed to response to these topical agents in daily practice.
Because topical TCIs show favorable outcomes for POD and because microbe involvement is discussed in POD etiology, agents with anti-inflammatory and anti-microbe characters would be benefit for POD treatment. For pediatric POD, safety of medications and treatments also should be carefully monitored. Among antibiotics, macrolides have immune modulating effects. Safety profiles of macrolides for pediatric patients are well monitored in treatment of chronic inflammatory airway diseases including chronic rhinosinusitis. We have used clarithromycin for treatment of refractory pediatric POD. In this study, we retrospectively investigated cases of pediatric POD treated with clarithromycin (CLR) and discuss safety and efficacy of CLR for pediatric POD.
Method and Materials
The retrospective study was conducted in Watanabe Dermatology and Plastic Surgery Clinic. This study was approved by the head of the institute. Patients gave consent to use their images. The medical records of pediatric patients with POD treated with CLR between January 2021 and February 2023 were reviewed. POD was diagnosed based on skin lesions with papules accumulated on nasolabial folds to angle of mouth and around mouth to chin. Patients had none or vary mild itchy on skin lesions.
Personal history, history of topical medication on face, adverse events during CLR treatment and outcomes after CLR treatment were reviewed from medical records. Outcomes were defined as very effective: Complete Responses (CR) in 4 weeks, effective: CR in 8 weeks, Partial Response (PR): improved but not achieved CR in 8 weeks and No Response (NR): no change or worsen. If POD reappeared after CR, cases were defined as a relapse.
In daily practice, pediatric patients were instructed to take 5 mg/kg of CLR twice daily (10 mg/kg/day) after meals. Patients were instructed to stop use Topical Glucocorticoid (TGC) and TCI if patients had treatment history of those. White petrolatum or topical moisturizer (heparinoid cream or ointment) were allowed to use for perioral lesions. Patients were instructed to revisit the clinic in a week or two weeks. Patients were also indicated to revisit the clinic when they had adverse events or worsened after CLR medication.
Results
During January 2021 and February 2023, 39 pediatrics were diagnosed as POD and received prescription of CLR. Among 19 females and 20 males, age distribution was from 1 to 15 years and average age was 5.3 ± 3.9 years (interquartile rage 2-9) (Fig. 1). Twelve (30.8%) had atopic dermatitis in treatment or in history (Table 1). Twenty-one (53.8%) used Topical Glucocorticoid (TGC) and/or Topical Calcineurin Inhibitor (TCI) on face. Reasons of facial use of TGC or TCI were atopic dermatitis (N=16) or perioral lesions without adequate diagnosis (N=9). Duration of facial topical use was diverse from less than a month (N=3) to more than 6 months (N=3). None of them improved POD by TGC and/or TCI.
Among 39 pediatric POD, 31 revisited the clinic and 8 did not (Fig.2). None of 31 experienced adverse events during CLR meditation. Among 31 cases who revisited the clinic, we selected 25 cases for efficacy evaluation and excluded 6 cases. Six cases stopped medication by their own reason and/or did not take medication regularly as prescribed (Fig. 2). Twenty-five cases included 13 females and 12 males and average age was 6.2 ± 4.2 years (interquartile rage 2-9) (Fig. 3). Twenty-three cases achieved Complete Response (CR) by CLR: 18 achieved CR in 4 weeks and 5 achieved CR in 8 weeks (Table 2). One case showed Partial Response (PR) by CLR and one case worsen after CLR administration with cessation of TCI. Among 23 CR cases, 7 cases (30%) had relapsed during 1.5 to 19 months after CR. All of relapse cases achieved CR by re-administration of CLR. Representative cases of CR and No Response (NR) are shown in Fig. 4 and 5, respectively (Fig. 4-7.
|
| + | – | |
Atopic Dermatitis | 12 | 27 | ||
TGC and/or TCI on Face | 21 | 18 | ||
Type Of Topical Use | TGC alone | 16 | ||
TCI alone | 3 | |||
TGC and TCI | 2 | |||
Reason Of Topical Use | Atopic dermatitis | 9 | ||
Perioral lesions | 11 | |||
Unknown | 1 | |||
Duration of Topical Use | Less than 1 month | 3 | ||
1-5 months | 4 | |||
More than 6 months | 3 | |||
Unknown | 1 | |||
TGCTopical Glucocorticoid, TCI: Topical Calcineurin Inhibitor | ||||
Table 1: Patients background.
Efficacy | Definition | N | Relapse (N) | Duration Until Relapse | Readministration of CLR |
Very effective | CR in 4 weeks | 18 | 4 | 1.5 – 7 months | Very effective to effective |
Effective | CR in 8 weeks | 5 | 3 | 5.5 – 19 months | Very effective to effective |
PR | Improved but not achieved CR in 8 weeks | 1 | – | – | – |
NR | No change or worsen | 1 | – | – | – |
CR: Complete Response, PR: Partial Response, NR: No Response, CLR: Clarithromycin, N: Number | |||||
Table 2: Efficacy of clarithromycin.
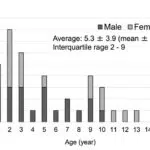
Figure 1: Age and gender distribution of pediatric perioral dermatitis. Age and gender distribution of 39 (19 females and 20 males) pediatric perioral dermatitis treated with clarithromycin during January 2021 and February 2023 are shown.

Figure 2: The study design to evaluate safety and efficacy of clarithromycin treatment for pediatric perioral dermatitis. During January 2021 and February 2023, 39 pediatrics were diagnosed as Perioral Dermatitis (POD) and received prescription of Clarithromycin (CLR). Excluding 8 cases who did not revisit the clinic, 31 were included for safety evaluation during CLR meditation. We selected 25 cases for efficacy evaluation because 6 cases stopped medication by their own reason and/or did not take medication regularly as prescribed.

Figure 3: Age and gender distribution of pediatric POD evaluated for clarithromycin efficacy. Data of total 25 (13 females and 12 males) were used for efficacy evaluation of clarithromycin treatment. Age and gender distribution of 25 pediatric perioral dermatitis are shown.

Figure 4: A representative case of pediatric perioral dermatitis treated very effectively by clarithromycin. A female case of 5 years old. She developed perioral papules a month before visiting our clinic. She has been treated with topical glucocorticoid in another clinic but did not improve lesions. (a): After 11 days of oral clarithromycin medication in our clinic, papular lesions diminished; (b): She did not relapse perioral lesions after stopping oral clarithromycin.

Figure 5: A representative case of pediatric perioral dermatitis treated very effectively by clarithromycin. A male case of 1 year old. He developed perioral papules a month before visiting our clinic. He has been treated with topical glucocorticoids in another clinic but did not improve lesions. (a): After 3 weeks of oral clarithromycin medication in our clinic, papular lesions diminished; (b): Total 4 weeks of oral clarithromycin medication was applied and he never relapsed perioral lesions.

Figure 6: A representative case of pediatric perioral dermatitis treated effectively by clarithromycin. A male case of 10 years old. He developed perioral papules three month before visiting our clinic. He has been treated with topical glucocorticoids and topical tetracycline in another clinic but did not improve lesions. (a): After 3 weeks of oral clarithromycin medication in our clinic, papular lesions diminished; (b): Total 5 weeks of oral clarithromycin medication was applied and he never relapsed perioral lesions.

Figure 7: A case of pediatric periorificial dermatitis worsen after clarithromycin treatment. A female case of 2 years old. She has atopic dermatitis and been treated with topical tacrolimus on face. She has developed perioral papules for 5 months. Although topical tacrolimus partially reduced papules, she repeated perioral papules and gradually increased papules on periorbital area. (a): After 14 days of oral clarithromycin medication with cessation of topical tacrolimus; (b): papular lesions increased.
Discussion
In this study, we reviewed 39 cases of pediatric POD who received a prescription of clarithromycin. We could follow 31 cases after CLR prescription and none of them had adverse events during CLR medication. Among 25 cases who regularly took medication and were evaluated for efficacy profile of CLR, 23 (92%) achieved CR in 8 weeks. Although 7 cases (30%) relapsed, all relapsed cases achieved CR by re-administration of CLR without any adverse events. So far, further relapse was not observed. Overall, oral CLR is safe and effective treatment for refractory pediatric POD.
Because POD is refractory and chronic dermatitis, treatment of POD is often challenging especially for pediatric patients. In the retrospective study evaluating efficacy and safety of TCIs pimecrolimus or tacrolimus, among 72 pediatric patients, CR was noted in 33 (68.8%) of 48 patients treated with TCI alone, in 9 (75%) of 12 patients treated with TCI and metronidazole and in 7 (77.8%) of 9 patients treated with TCI and a systemic antibiotic included azithromycin, erythromycin or minocycline [9]. They reported that the median time to PR or CR was 14 days (interquartile range, 7-60). In the combination of topical TCI and topical/oral metronidazole, a retrospective study reported that 14 (58.3%) and 10 (41.7%) of 24 pediatric periorificial dermatitis achieved CR and PR, respectively [10]. The median treatment period was 5.5 months in the study. One patient reported mild diarrhea after taking 500 mg per day of oral metronidazole, the reduction to 250 mg per day eliminated symptoms. We observed CLR suppressed symptoms of pediatric POD smoothly. Among 23 cases (92%) achieving CR by CLR, 18 (72%) achieved CR in 4 weeks and 5 (20%) achieved CR in 8 weeks. Because TCI, as well as TGC, could exacerbate or induce perioral dermatitis and rosaceiform eruptions, our patients were instructed to stop TCI and TGC and to use only moisturizer when CLR was prescribed [11-13]. Although one case worsened periorificial dermatitis after TCI cessation, others well tolerated transient exacerbation after TCI and TGC cessation. Because more than 90% of our patients achieved CR by CLR alone, CLR is worthy to treat pediatric POD without other topical medication. Therefore, oral CLR is an effective and considerable treatment for pediatric POD instead of TCI and other treatments.
Under the pandemic of COVID-19, it has been recommended or mandatory to wear a face mask in social areas including schools since 2019. Reports of facial dermatoses associated with facial masks have increased since 2020: including contact mask-induced acne ‘maskne’ perioral dermatitis and Koebner phenomenon [14-21]. In the retrospective cohort studies from groups of Milan, Italy and New York, USA, they independently reported that incident of POD is increased after 2019 [22,23]. We also noticed increase in pediatric POD patients after the outbreak of COVID-19. POD is associated with skin dysbiosis [1,2]. Although direct connection of SARS-Cov2 to POD pathogenesis have not been proposed in our best knowledge, microbiome dysbiosis have been observed with facial masks [18]. Facial mask also changes local skin conditions; skin temperature, hydration, sebum secretion and blood flow represented by facial redness [24]. POD induced in users of CPAP (continuous positive airway pressure) devises are also reported [25,26]. Therefore, POD induction and exacerbation should be considered in cases of patients with facial mask or facial occlusive devices.
Conclusion
There are several limitations of the study. Because this study was retrospective study, controls and placebo groups were not applied in the analysis. Although we did not observe any adverse events, we could not confirm safety of 8 cases who did not revisit the clinic. Long term effects of oral CLR for children cannot be discussed in this study. As we observed 7 cases relapsed POD, we do not suggest that CLR achieve absolute cure for the refractory and chronic inflammation of POD. Because topical erythromycin creams are not on the market in Japan, we do not have experiences of topical macrolide for pediatric POD. Although topical macrolide might be safer, we cannot compare the efficacy between topical and oral macrolides. Despite of these limitations of the retrospective study, we concluded that oral CRL is a worth treatment to suppress symptoms of pediatric POD.
Conflict of Interests
Yuko Watanabe does not have any COI relating to the article. Kenshi Yamasaki has received honorariums of lecturing from Maruho, AbbVie, Sato Pharmaceutical, Eli Lilly, UCB, Taiho Pharmaceutical, Sun Pharma Japan, Nippon Boehringer Ingelheim, Novartis and Eisai and has served a consultant of Kao and Pola Chemical Industries. Kenshi Yamasaki is an Editorial Board member of The Journal of Dermatology and a co-author of this article. To minimize bias, they were excluded from all editorial decision-making related to the acceptance of this article for publication.
References
Research Article
Received Date: 15-01-2024
Accepted Date: 10-02-2024
Published Date: 17-02-2024
Copyright© 2024 by Yamasaki K, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Yamasaki K, et al. Oral Clarithromycin Therapy for Perioral Dermatitis in Children: A Retrospective Case-series Study. J Dermatol Res. 2024;5(1):1-8.

Figure 1: Age and gender distribution of pediatric perioral dermatitis. Age and gender distribution of 39 (19 females and 20 males) pediatric perioral dermatitis treated with clarithromycin during January 2021 and February 2023 are shown.

Figure 2: The study design to evaluate safety and efficacy of clarithromycin treatment for pediatric perioral dermatitis. During January 2021 and February 2023, 39 pediatrics were diagnosed as Perioral Dermatitis (POD) and received prescription of Clarithromycin (CLR). Excluding 8 cases who did not revisit the clinic, 31 were included for safety evaluation during CLR meditation. We selected 25 cases for efficacy evaluation because 6 cases stopped medication by their own reason and/or did not take medication regularly as prescribed.

Figure 3: Age and gender distribution of pediatric POD evaluated for clarithromycin efficacy. Data of total 25 (13 females and 12 males) were used for efficacy evaluation of clarithromycin treatment. Age and gender distribution of 25 pediatric perioral dermatitis are shown.

Figure 4: A representative case of pediatric perioral dermatitis treated very effectively by clarithromycin. A female case of 5 years old. She developed perioral papules a month before visiting our clinic. She has been treated with topical glucocorticoid in another clinic but did not improve lesions. (a): After 11 days of oral clarithromycin medication in our clinic, papular lesions diminished; (b): She did not relapse perioral lesions after stopping oral clarithromycin.

Figure 5: A representative case of pediatric perioral dermatitis treated very effectively by clarithromycin. A male case of 1 year old. He developed perioral papules a month before visiting our clinic. He has been treated with topical glucocorticoids in another clinic but did not improve lesions. (a): After 3 weeks of oral clarithromycin medication in our clinic, papular lesions diminished; (b): Total 4 weeks of oral clarithromycin medication was applied and he never relapsed perioral lesions.

Figure 6: A representative case of pediatric perioral dermatitis treated effectively by clarithromycin. A male case of 10 years old. He developed perioral papules three month before visiting our clinic. He has been treated with topical glucocorticoids and topical tetracycline in another clinic but did not improve lesions. (a): After 3 weeks of oral clarithromycin medication in our clinic, papular lesions diminished; (b): Total 5 weeks of oral clarithromycin medication was applied and he never relapsed perioral lesions.

Figure 7: A case of pediatric periorificial dermatitis worsen after clarithromycin treatment. A female case of 2 years old. She has atopic dermatitis and been treated with topical tacrolimus on face. She has developed perioral papules for 5 months. Although topical tacrolimus partially reduced papules, she repeated perioral papules and gradually increased papules on periorbital area. (a): After 14 days of oral clarithromycin medication with cessation of topical tacrolimus; (b): papular lesions increased.
| + | – | ||
Atopic Dermatitis | 12 | 27 | ||
TGC and/or TCI on Face | 21 | 18 | ||
Type Of Topical Use | TGC alone | 16 | ||
TCI alone | 3 | |||
TGC and TCI | 2 | |||
Reason Of Topical Use | Atopic dermatitis | 9 | ||
Perioral lesions | 11 | |||
Unknown | 1 | |||
Duration of Topical Use | Less than 1 month | 3 | ||
1-5 months | 4 | |||
More than 6 months | 3 | |||
Unknown | 1 | |||
TGCTopical Glucocorticoid, TCI: Topical Calcineurin Inhibitor | ||||
Table 1: Patients background.
Efficacy | Definition | N | Relapse (N) | Duration Until Relapse | Readministration of CLR |
Very effective | CR in 4 weeks | 18 | 4 | 1.5 – 7 months | Very effective to effective |
Effective | CR in 8 weeks | 5 | 3 | 5.5 – 19 months | Very effective to effective |
PR | Improved but not achieved CR in 8 weeks | 1 | – | – | – |
NR | No change or worsen | 1 | – | – | – |
CR: Complete Response, PR: Partial Response, NR: No Response, CLR: Clarithromycin, N: Number | |||||
Table 2: Efficacy of clarithromycin.