Hetvi Solanki1, Vincent S Gallicchio1*
1Department of Biological Sciences, College of Science, Clemson University, Clemson, SC, 29636, USA
*Correspondence author: Vincent S Gallicchio, Department of Biological Sciences, College of Science, Clemson University, Clemson, SC, 29636, USA; Email: [email protected]
Published Date: 30-09-2023
Copyright© 2023 by Solanki H, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Left Ventricular Hypertrophy is a multifactorial condition involving genetic and signaling pathways. Current recommendations for treating hypertensive LVH include ACE inhibitors, RAS and CCBs. Despite numerous drug therapies for ventricular hypertrophy, patients often deteriorate and experience a decline in ventricular function ultimately leading to heart failure. For this reason, alternative therapies must be investigated. Stem cells possess the potential to divide at a high capacity, self-renew and differentiate into numerous cardiac cell lineages. Furthermore, stem cells are connected to ventricular remodeling and are vital in maintaining the homeostasis of the heart. Further examination of the role of stem cells in LVH could result in the development of targeted therapeutic interventions for prevention or reversal of the phenomenon.
Keywords: Stem Cells; Left Ventricular Hypertrophy; Cardiac; Heart Failure
Abbreviations:
ACE: Angiotensin-Converting Enzyme; bFGF: basic Fibroblast Growth Factor; BP: n-Butylidenepthalide; CCB: Calcium Channel Blockers; CD: Cluster of Differentiation; CSC: Cardiac Stem Cells; EGFP: Enhanced Green Fluorescent Protein; GATA4: GATA Binding Protein 4; hADSC: human Adipose Derived Stem Cells; HCM: Hypertrophic Cardiomyopathy; hUCB: human Umbilical Cord Blood; IGF-1: Insulin-Like Growth Factor; IL: Interleukin; LV: Left Ventricle; LVH: Left Ventricular Hypertrophy; MI: Myocardial Infarction; MSC: Mesenchymal Stem Cells; PCR: Polymerase Chain Reaction; RAS: Renin Angiotensin System; ROS: Reactive Oxygen Species; SHR: Spontaneously Hypertensive Rats; STAT: Signal Transducer and Activator of Transcription; TGF-B1: Transforming Growth Factor B1; TNFa: Tumor Necrosis Factor Alpha; TRPC1: Transient Receptor Potential Canonical 1; VEGF: Vascular Endothelial Growth Factor; VSEL-SC: Very Small Embryonic-Like Stem Cells
Introduction
Left ventricular hypertrophy is a multifactorial condition involving genetic and signaling pathways [1]. Hypertrophy can cause a decline in ventricular function and ultimately heart failure [1]. A three-fold increase in the occurrence of Ventricular Tachycardia (VT) and Ventricular Fibrillation (VF) is observed in patients with hypertensive LVH [2]. Current recommendations for treating hypertensive LVH include ACE inhibitors, RAS and CCBs [2,3]. Despite numerous drug therapies for ventricular hypertrophy, patients often deteriorate. For this reason, alternative therapies must be investigated [4].
Stem cells and progenitor cells exhibit high proliferative potential and secrete numerous growth factors, cytokines and microRNAs which play vital roles in cardiac remodeling, cell differentiation and neovascularization [1]. In many cardiovascular conditions with pathologies like LVH, such as myocardial infarction, hematopoietic stem cells, endothelial progenitor cells and mesenchymal stem cells are rapidly mobilized [1]. Cardiac stem cells can regulate myocyte turnover and myocardial recovery post-injury [1]. Bone marrow-derived stem cells can improve cardiac function and survival through the secretion of proangiogenic factors that stimulate endogenous neovascularization and differentiation into functional adult myocytes and vascular cells [1]. MSCs can contribute to neovascularization, fibrosis and ventricular wall remodeling [5]. Further examination of the role of stem cells in LVH could result in the development of targeted therapeutic interventions for prevention or reversal of the phenomenon [1].
Epidemiology and Pathophysiology
Left Ventricular Hypertrophy is a multifactorial condition involving genetic and signaling pathways [1]. Increased chances of stroke, ventricular dysfunction, ventricular arrhythmias and sudden death are all linked to cardiac hypertrophy [6]. Cardiac hypertrophy which consists of an increase in left ventricular mass and thickening of the ventricular wall, is an adaptive response to counteract increased workload [7]. LVH can take on two forms: physiological or pathological myocardial hypertrophy [1].
Physiological myocardial hypertrophy often occurs in healthy conditions such as in athletes or pregnant women. Normal cardiac structure is maintained despite physiological hypertrophy. Based on experimental studies primarily c-kit+ Lin- cells increase in the heart during exercise and Sca-1+ – Lin- may also be stimulated. Limited data is available for pregnancy and the postnatal heart [1].
Pathological cardiac hypertrophy is most often caused by pressure overload which ultimately results in an increase in wall thickness and concentric hypertrophy of the left ventricle as a compensatory mechanism in order to maintain the ventricular ejection fraction under conditions of increased peripheral resistance. Volume overload can also cause pathological cardiac hypertrophy. Complex gene reprogramming in cardiac cells often accompanies hypertrophy. LVH entails an increase in the number of sarcomeres, perivascular and interstitial connective tissue and ground substance and the capillary and nerve networks. A key factor in the pathophysiology of pathological hypertrophy is the expression of fetal genes alpha-skeletal actin and beta-myosin heavy chains [1].
Why Treatments Have Failed
Current recommendations for treating hypertensive LVH include ACE inhibitors, RAS and CCBs [2,3]. As of right now, ACE inhibitors present themselves as the most potent treatment for LVH [3]. Beta blockers, on the other hand, exhibit an insufficient capacity to reverse LVH and therefore are not recommended as a treatment [2]. Despite numerous drug therapies being available for ventricular hypertrophy, patients often deteriorate. For this reason, alternative therapies must be investigated [4].
Discussion
Stem cells possess the potential to divide at a high capacity, self-renew and differentiate into numerous cardiac cell lineages [1]. Furthermore, stem cells are connected to ventricular remodeling and are vital in maintaining the homeostasis of the heart [1]. Based on a portion of the reservoir of stem cells and progenitor cells in myocardial tissue, the human heart has an intrinsic regenerative potential and is in continuous turnover [1]. Further examination of the role of stem cells in LVH could result in the development of targeted therapeutic interventions for prevention or reversal of the phenomenon [1].
Use of Stem Cells to Treat LVH
Stem cells and progenitor cells exhibit high proliferative potential and secrete numerous growth factors, cytokines and microRNAs which play vital roles in cardiac remodeling, cell differentiation and neovascularization [1]. Stem and progenitor cells are activated in LVH and perform a regulatory role in myocardial repair via their contribution to the renewal of adult mammalian cardiomyocytes in cases of myocardial injury or pressure/volume overload [1]. To interact with myocardial cells, the precursor cells migrate from the epicardium to the myocardium. The formation of nearly all cardiac structures in myocardial hypertrophy involves migratory cells. In many cardiovascular conditions with a pathology similar to LVH, such as myocardial infarction, hematopoietic stem cells, endothelial progenitor cells and mesenchymal stem cells are rapidly mobilized [1].
Cardiac stem cells can regulate myocyte turnover and myocardial recovery post-injury. CSCs are stored in niches which are favorable microenvironments. Leaving the niche can trigger their growth, migration and commitment into myocardial tissue [1].
It has been demonstrated that survival and cardiac function can be improved by bone marrow-derived stem cells through the secretion of proangiogenic factors that stimulate endogenous neovascularization and differentiation into functional adult myocytes and vascular cells [1]. Bone marrow-derived stem cells behave differently in cardiovascular diseases which is why their pathophysiology is not well-understood yet [1].
MSCs can contribute to neovascularization, fibrosis and ventricular wall remodeling [5]. A recent study showed that in hypertensive patients who presented an increased expression of myocardin and GATA4 in the peripheral blood mononuclear cell fraction, which are associated with blood pressure levels and LVH, the presence of mesenchymal progenitor cells was implied which could possibly be intended to differentiate into cardiac series cells [8]. MSC’s role in the pathophysiology of arterial hypertension needs to be further investigated (Fig. 1) [8].
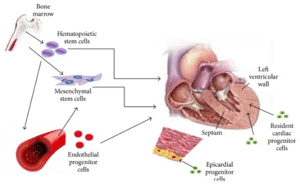
Figure 1: Numerous types of stem cells that partake in myocardial regeneration and left ventricular hypertrophy remodeling are depicted [1].
Animal Trials
A study using animals (197 mice) was conducted. 49 of them were used in the MI study. 142 EGFP transgenic mice acted as bone marrow donors for cell isolation. Myocyte area was determined using 6 mice. 8 mice were excluded for technical reasons. 5 died in the early-infarction period and within 72 hours of intramyocardial injection administration, 10 more died. A 30-minute coronary occlusion, followed by reperfusion was performed on mice. 48 hours later, an intramyocardial injection was administered with one of the following: vehicle, [1×10]^5 EGFP-labeled expanded untreated VSEL-SCs or [1×10]^5 EGFP-labelled expanded VSEL-SCs pre-incubated in a cardiogenic medium. The cardiogenic medium contains Transforming Growth Factor B1 (TGF-B1), Vascular Endothelial Growth Factor (VEGF), Basic Fibroblast Growth Factor (bFGF) and Insulin-Like Growth Factor-1 (IGF-1). Group 1, which included 11 mice, received the vehicle. Group 2 consisting of 7 mice received the [1×10]^5 EGFP-labeled expanded untreated VSEL-SCs. 8 mice in group 3 received [1×10]^5 EGFP-labelled expanded VSEL-SCs pre-incubated in a cardiogenic medium. 48 hours after injecting the cells and 35 days after the MI, echocardiograms were performed. 35 days post-MI, it was observed that the mice treated with group 3 VSEL-SCs exhibited improved global and regional LV systolic function and decreased LV hypertrophy when compared with vehicle-treated control mice. No significant benefits were observed in the group 2 mice. No tumor formation was observed. With exposure to cardiomyogenic growth factors and cytokines before transplantation, VSEL-SCs expanded in culture retain the ability to aid in LV dysfunction and reperfused MI. Cardiomyocytic or endothelial differentiation was rarely observed in the transplanted VSEL-SCs which implies that paracrine mechanisms mediate the effects of the pre-incubated VSEL-SCs. Adult bone marrow cell transplantation is associated with improved structure and function of the left ventricle. Further investigation is needed to determine the specific molecular mechanisms in which pre-incubated VSEL-SCs enhance cardiac repair [9].
A rat model of pressure overload hypertrophy was used to investigate the effects of delivering MSCs via intracoronary injection on left ventricular reverse remodeling, hemodynamic performance, exercise capacity and systemic inflammation. Aortic banding followed by echocardiographic scanning was performed on Sprague-Dawley rats. After a 25% decrease in fractional shortening occurred, the rats were randomized into two groups, one receiving an intracoronary injection of MSCs (n=28) and one receiving a phosphate-buffered saline solution (n=20). On post-op days 7, 14, 21 and 28 hemodynamic and echocardiographic assessment and a swim test to exhaustion were performed and measurements of inflammatory markers were taken. Systolic function and time to exhaustion improved via injection of MSCs in comparison with the control group. A significant decrease was observed in levels of IL1 and IL6, Tumor Necrosis Factor-Alpha (TNFα) and brain natriuretic peptide-32 in the MSC group. At 21 and 28 days, an improvement in left ventricular fractional shortening occurred. In addition, at 28 days, left ventricular end-systolic and end-diastolic diameters improved. Further investigation is needed to determine whether high-dosages or repeated-dosages of MSCs are more effective, what the best delivery method is, whether MSCs are the optimal cell population, if cells from older donors are as potent as cells from younger donors and if inductors of differentiation are safe [10].
In another study, age-matched male Fischer 344 rats were used to avoid confounding factors. Isolation of nonhematopoietic CD45-Lin- cells from human umbilical cord cells was performed. To characterize this subpopulation, quantitative PCR and flow cytometry were used. 30-minute coronary occlusion was performed on 72 rats. An intramyocardial injection of either the vehicle or hUCB CD45-Lin- cells was administered 48 hours later. Improved left ventricular function, decreased LVH, greater preservation of viable myocardium in the infarct zone and increased left ventricular remodeling was observed in the CD45-Lin- cell-treated rats. Injection of hUCB CD45-Lin- cells modulated molecular pathways that regulate myocardial fibrosis, cardiomyocyte apoptosis, angiogenesis and inflammation in postinfarct ventricular myocardium. Patient age and comorbid conditions adversely affect the quantity and function of bone marrow cells. Allogenic cells possess the potential for rejection. hUCB and progenitor cells are multipotent and immunologically naïve which allows them to overcome the limitations presented by other cells. hUCB remain viable and retain engrafting capability even after long periods of cryopreservation. Persistence of a low frequency of transplanted human cells in the hearts of rats suggested that the observed reparative benefits are a result of paracrine mechanisms [11].
Twelve-week-old male Spontaneously Hypertensive Rats (SHRs) randomly received an injection in the right hamstring of either vehicle, clinical-grade hADSCs and BP(n-butylidenepthalide)-preconditioned hADSCs for 8 weeks. The ratio of LV weight to tibia, cardiomyocyte cell size and collagen deposition independent of hemodynamic changes, decreased in naïve hADSCs as compared to untreated SHRs. Myocardial ROS (reactive oxygen species) production was attenuated and increased p-STAT3 levels also occurred. BP-preconditioned hADSCs resulted in a greater decrease of ROS and LVH while also causing an increase in local hADSC engraftment, STAT3 phosphorylation, STAT3 activity, STAT3 nuclear translocation, myocardial IL-10 levels and percentage of M2 macrophage infiltration. Cardiac hypertrophy even at an established phase of hypertension can be reversed via remote transplantation of hADSCs. Migration of remotely transplanted hADSCs to the heart did not occur. hADSCs regulated macrophage transformation thus attenuating ventricular hypertrophy. LVH can be attenuated in a safe and non-invasive manner by remote transplantation of hADSCs. hADSC-related activation of the STAT3 pathway that contributes to attenuated cardiac hypertrophy may be related to the phenotypic shift of the macrophage from M1 to M2 [6].
Immunological and histochemical changes in the left ventricular that occur because of hypertension can be improved significantly via MSC therapy. This finding was confirmed by a study conducted on adult male albino rats. The rats were divided into 2 groups. Group 1 functioned as the control. Group 2 served as the experimental group and was further divided into subgroup 2a which included hypertensive rats and group 2b which included rats receiving stem cell therapy. Processing of the left ventricles for light and electron microscope took place and Mallory’s trichrome immunostaining was performed for caspase-3 and desmin. MSCs were found to be an effective therapy for hypertensive cardiomyopathy [12].
Clinical Trials
A study was conducted to investigate MSC circulation in patients with essential hypertension. 24 patients with untreated essential hypertension and 19 healthy patients were included. MSCs in the peripheral blood were measured via flow cytometry as a population of CD45-/CD34-/CD90+ and CD45-/CD34-/CD105+ cells. It was found that hypertensive patients had increased levels of circulating CD45-/CD34-/CD90+ in comparison with the controls (0.0069% +/- 0.012% vs 0.00085% +/- 0.0015% respectively). Left ventricular mass index was found to be positively correlated with the levels of CD45-/CD34-/CD90+ circulating cells. There was no significant difference in circulating CD-45/CD34-/CD105+ between hypertensive and normal patients’ peripheral blood. This study had several limitations. There was a small sample size which limits data collection. In addition, there was a lack of mechanistic explanation of the data in terms of whether the MSC populations studied differed between groups or not. Also, the fate of the increased number of circulating CD45-/CD34-/CD90+ cells was unknown, they could have differentiated into myocardial or nonmyocyte cardiac cells. Lastly, the effects of antihypertensive medication on the mobilization of bone marrow derived MSCs were not investigated [8-12].
Conclusion
Left Ventricular Hypertrophy is a multifactorial condition involving genetic and signaling pathways. Increased chances of stroke, ventricular dysfunction, ventricular arrhythmias and sudden death are all linked to cardiac hypertrophy. Despite numerous drug therapies for ventricular hypertrophy, patients often deteriorate. For this reason, alternative therapies must be investigated. Stem cells and progenitor cells exhibit high proliferative potential and secrete numerous growth factors, cytokines and microRNAs which play vital roles in cardiac remodeling, cell differentiation and neovascularization. Cardiac stem cells can regulate myocyte turnover and myocardial recovery post-injury. Bone marrow-derived stem cells can improve cardiac function and survival through the secretion of proangiogenic factors that stimulate endogenous neovascularization and differentiation into functional adult myocytes and vascular cells. MSCs can contribute to neovascularization, fibrosis and ventricular wall remodeling. Further investigation is needed regarding many aspects of stem cell therapy as a treatment for LVH. The specific molecular mechanisms in which pre-incubated VSEL-SCs enhance cardiac repair must be further investigated. In addition, further investigation is needed to determine whether high-dosages or repeated-dosages of MSCs are more effective, what the best delivery method is, whether MSCs are the optimal cell population, if cells from older donors as potent as cells from younger donors and if inductors of differentiation are safe.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- Marketou ME, Parthenakis F, Vardas PE. Pathological left ventricular hypertrophy and stem cells: current evidence and new perspectives. Stem Cells Int. 2016;2016.
- Koracevic G, Stojanovic M, Lovic D, Zdravkovic M, Sakac D. Certain beta blockers (eg, bisoprolol) may be reevaluated in hypertension guidelines for patients with left ventricular hypertrophy to diminish the ventricular arrhythmic risk. J Human Hypertension. 2021;35(7):564-76.
- Messerli FH, Soria F, Aristizabal D. Left ventricular hypertrophy: should it be reduced? Clin Cardiol. 1993;16(S2):15-20.
- McCarty MF. Nutraceutical, dietary and lifestyle options for prevention and treatment of ventricular hypertrophy and heart failure. Int J Molecular Sciences. 2021;22(7):3321.
- Marketou ME, Parthenakis FI, Kalyva A, Pontikoglou C, Maragkoudakis S, Kontaraki JE, et al. Circulating mesenchymal stem cells in patients with hypertrophic cardiomyopathy. Cardiovascular Pathol. 2015;24(3):149-53.
- Lee TM, Harn HJ, Chiou TW, Chuang MH, Chen CH, Chuang CH, et al. Remote transplantation of human adipose-derived stem cells induces regression of cardiac hypertrophy by regulating the macrophage polarization in spontaneously hypertensive rats. Redox Biol. 2019;27:101170.
- Tang L, Yao F, Wang H, Wang X, Shen J, Dai B, et al. Inhibition of TRPC1 prevents cardiac hypertrophy via NF-κB signaling pathway in human pluripotent stem cell-derived cardiomyocytes. J Mol Cellular Cardiol. 2019;126:143-54.
- Marketou ME, Parthenakis FI, Kalyva A, Pontikoglou C, Maragkoudakis S, Kontaraki JE, et al. Increased mobilization of mesenchymal stem cells in patients with essential hypertension: the effect of left ventricular hypertrophy. J Clin Hypertension. 2014;16(12):883-8.
- Zuba‐Surma EK, Guo Y, Taher H, Sanganalmath SK, Hunt G, Vincent RJ, et al. Transplantation of expanded bone marrow‐derived very small embryonic‐like stem cells (VSEL‐SCs) improves left ventricular function and remodelling after myocardial infarction. J Cellular Mol Med. 2011;15(6):1319-28.
- Molina EJ, Palma J, Gupta D, Torres D, Gaughan JP, Houser S, et al. Improvement in hemodynamic performance, exercise capacity, inflammatory profile and left ventricular reverse remodeling after intracoronary delivery of mesenchymal stem cells in an experimental model of pressure overload hypertrophy. J Thoracic and Cardiovascular Surg. 2008;135(2):292-9.
- Zhao L, Cheng G, Choksi K, Samanta A, Girgis M, Soder R, et al. Transplantation of human umbilical cord blood-derived cellular fraction improves left ventricular function and remodeling after myocardial ischemia/reperfusion. Circulation Res. 2019;125(8):759-72.
- Khater NA, Selim SA, Abd El-Baset SA, Abd El Hameed SH. Therapeutic effect of mesenchymal stem cells on experimentally induced hypertensive cardiomyopathy in adult albino rats. Ultrastructural Pathol. 2017;41(1):36-50.
Article Type
Review Article
Publication History
Received Date: 26-08-2023
Accepted Date: 23-09-2023
Published Date: 30-09-2023
Copyright© 2023 by Solanki H, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Solanki H, et al. Use of Stem Cells to Treat Left Ventricular Hypertrophy. J Reg Med Biol Res. 2023;4(3):1-6.

Figure 1: Numerous types of stem cells that partake in myocardial regeneration and left ventricular hypertrophy remodeling are depicted [1].


