Prithiv Kumar KR1*
1Director, Principle Scientist- Poichyadical Stem Cell Centre for Research and Development (POSCERD), Chicago, USA and CEO, La’ zer Group of Companies, Chicago, USA
*Corresponding Author: Prithiv Kumar KR, Director, Principle Scientist- Poichyadical Stem Cell Centre for Research and Development (POSCERD), Chicago, USA and CEO, La’ zer Group of Companies, Chicago, USA, Egypt; Email: [email protected]
Published Date: 31-08-2022
Copyright© 2022 by Kumar PKR. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Creutzfeldt Jakob Disease (CJD) is incurable and fatal neurodegenerative disease. The treatment in human is cell based models for prion disease. An induced pluripotent stem cell based models of human cerebral organ has shown to cure CJD prions. This model has new screening methodology for drug induced candidates in certain genetic background. Using anti-prion disease, first ever model was screened for drug induced patients. Pentason polysulfate delayed methodology along with prophylactic has been a remedy for treatment. These are the first regenerative treatment that can concur results for CJD.
Keywords
Neurodegenerative Disease; Prior Protein; Brain Structure; Sinusitis
Physiology
Normal cellular Prior Protein (PrP) is found in the membrane of cells throughout the body. The changes of PrP causes this CJD [1]. The disease causing Scrapie in the PrP self-propagates and accumulated throughout the brain. The isoform changes structurally in native prion protein which is believed to have infective capacity transforming normal prion to abnormal one and accumulated in brain to form degenerative disease [2].
Clinical Features
Rapidly progressive dementia and multi focal neurological diseases such as visual disturbances, cerebellar, pyramidal, extrapyramidal signs, and myoclonus are the several clinical features of CJD. Mostly this disease occurs due rapid progression in functional and cognitive impairment towards kinetic mutism in last stage (Fig. 1) [3].

Figure 1: Above figure shows CJD and its symptoms and impact on the brain [3].
Introduction
Neuro degenerative diseases generally have greater characteristics and are unique in each other’s perspective. In terms of intro or extra cellular accumulation of specific protein, it aggregates and accumulates in the Central Nervous System (CNS). These proteins have a predominant beta sheets form, resulting in multiple tissue storage which then aggregates in brain, leading to neuronal dysfunction and neuro degeneration [4].
CJD is a common prion disease that comprises of four types such as sporadic, genetic, variant and acquired, each with seemingly distant aetiologies. They along with other neurogenerative diseases can coexist if alpha, beta and tau sheets proteins are present, then the pathology is not as usual as sporadic in genes of the brain structure [5]. Misfold of tau protein aggregate is related to ageing of brain and is a common pathology causing cognitive impairment. Furthermore there are signs of lower frequency of Apolopoprotein E, (APOE) major genetic risk factor involving baplotype of Microtubule Associated Protein Tau (MAPT) which is less than 10% in consistency (Fig. 2,3).

Figure 2: The scan report shows brain manifested with CJD [5].

Figure 3: The above image shows prion infusion and its transmission [6].
Our goal is to identify the prion and its gene movement using genetic studies along with its accumulation factor and correlate with other neurodegenerative diseases without any comorbid proteinopathies and identify its uniqueness in curing [7].
Cases with CJD- Procedures for Test and Cure
All the procedures involving human participants were in accordance with ethical standards and institutional and/or national and regional ethical research committee along with terms of Helsinki Declaration (as revised 2013). All relevant consent was obtained before publication.
First case had hypertension, gastroesophageal reflux, and cervical spinal stenosis. No family history was reported. On physical exam the patient exhibited stuttered speech, lack of concentration, numbness, headache, light-headedness, failed finger to nose test, but patients cranial nerves were intact, CT scan did not show any contrast to acute abnormalities.in admission, patient was diagnosed with binocular horizontal diplopia and discharged for follow up with ophthalmologist for symptoms. The patients CT brain showed generalized cerebral and cerebellar atropic changes and left sided sphenoid sinusitis [8].
Image 1 reports mild cerebral volume loss without acute intracranial pathology. EEG when obtained displayed sharp wave complexes and triphasic waves. These results are consistent when compared with encephalopathy. Image 2- Patients CSF showed hazy and red in appearance, White Blood Cells (WBC) 7 nano litres, Red Blood Cells (RBC) 2000 nano litres and polynuclear WNCs 65% lymphocytes 22%, monocytes 13%, total protein 68 mg/dL, glucose 68 mg/dL. His serum WNC was 12.5*10^3/mm and rest lab results showing normal signs (Fig. 4-6) [9].

Figure 4: CJD showing sharp wave complexes [9].

Figure 5: Pattern of cortical changes in CJD [9].

Figure 6: Electroencephalogram (EEG) demonstrates typical sCJD features described in the literature. Initially patient had periodic slow wave complexes. As his disease progressed, characteristic bilateral synchronous periodic epilentiform discharges were seen as repetitive pattern of trio basic waves approximately 1-1.5 seconds apart [10].
Patient was started with treatment for infection. Levertiacetum was prescribed twice a day for seizure prophylaxis and used benzodiazepines sparingly for severe myoclonus. For one week high dose of methylprednisolone and intravenous immunoglobulin was given to treat possible autoimmune encephalitis. On constant repeats of EEG, patient emphased on Bilateral Synchronous Periodic Epileptiform Discahrges (BiPEDs) suggesting CJD [11].
Second case was referred to a memory clinic for 5 month evaluation history of progressive dementia. The initial symptoms were memory loss, feeling odd anorexia and unintentional weight loss. Short term memory and functional disabilities were very common with the patient. There was no myocardial infarction or leukemia, nor any family history of dementia or prion disease. Patients physical examination was significant for preservaration, anomie aphasia, alexia, agnosia and apraxia. Muscle tone showed normal signs, reflexes were symmetric and no Babinski reflexes. But the patient was unable to perform complicated tasks or minimum mental tasks. Lab report showed normal signs [11].
Cerebrospinal Fluid (CSF) studies were performed. Cell counts, glucose, and protein were within limits. Toxoplasma gondii, Bartonelli DNA, venereal disease research lab test and Lyme antibodies were negative. Protein 14-3-3 level was also normal, limits less than 1.0 ng/mL with normal range of less than 1.5 ng/mL. A non-contrast Magnetic Resonance Imaging (MRI) of her brain was significant for global parenchyma loss. Diffused weighted images showed restricted cortical diffusion in the cingulate gyrus and also in the bilateral parietal and posterior temporal lobes. Am EEG showed left temporal slowing with diffusely slowed and disorganised background and there were no periodic discharges noted (Fig. 7,8) [12].

Figure 7: Hyperintense signal in cingulate gyrus.

Figure 8: Restricted cortical diffusion.
The patient is consistent with CJD. Definitive diagnosis can be confirmed after western blot test. Pathogenic prion protein deposition in brain is seen and periodic sharp rise in EEG [12].
Prion Disease Cure Using Stem Cells
Antibody- mediated therapy targets PrP has been showing results in our experiments in-vitro. The antibodies bind to PrP sites and attenuate the availability for conversion into abnormal prion conformers to normal prion conformers. This approach has been promising so far, in delivering the antibodies via blood brain brain barrier and also immunological pitfalls associated with tolerance of PrP that is widely expressed in the immune system. Another approach was made, targeting inflammatory mediators using follicular dendritic cells. These cells are known for PrPs accumulations, neutralisation of the lymphotoxin beta receptor pathway and by administration for solvable protein blocks, these follicular dendritic cells mature and prevents scrapie neuro invasion. Conversion of PrP is a possible event in our experiments. It was the core in our pathology. Quinacrine (antibiotic drug) were found to be efficacious inhibitors of PrPs formation in vitro. The compounds act as mediator and try rearranging the cholesterol into intercellular compartment giving boost to plasma membrane. Protein folding is another event in the prion disease pathology hence we use pharmacological agents to halt the folded state of PrP. These agents allow reduction of protein misfolding and reduce progression of CJD. Recently tetranyrrole compound displayed significant anti prion activity by binding a folded domain of human PrP (Fig. 9,10) [13].

Figure 9: Promising method for prion disease cure [13].

Figure 10: Inhibition of gene expression and degradation of PrP [14].
RNA Working On Prion Sites
RNA interference exploits the endogenous cells and act as a gene slicing machinery and it recognises a potential target to inhibit the PrPC expression in neurobalstma cells and prevents the build-up of abnormal PrP conformers in scrapie infected cells. Apoptosis of neuronal cells is the central body in prion diseases. Neuronal cells in vitro undergoes apoptosis when incubated in the presence of , they are mimicked by PrP peptide fragment. They accumulate calcium sites that results in PrP formation. Flupirtine drug helps in reduction of calcium substitutes in PrP that antagonize the receptor channel of Ca2+, Na3+ and K+. In our experiment serotonin also helps Indy regulation of prion disease (Fig. 11). They alter the 5HT and 5HT regulator bindings in prions. PrP plays a minor role in binding 5HT receptor that couples to G-protein. In many of our experiments, 5HT drugs helped in solving pharmacological therapies [14].

Figure 11: Prion replication in endogenous adult neural cells [15].
Future Possible Therapies
Embryonic stem cells and neuronal precursors are known to migrate towards sites of brain damage and can differentiate into specific neuronal cell types. These are still in our experimental phase, there can be hope to attenuate this disease symptoms and give cure for CJD [15].
Conflict of Interest
The authors declare that they have no conflicts of interest to disclose.
References
- Department of Health and Human Services, Centers for Disease Control and Prevention, CJD (Creutzfeldt – Jakob disease, Classic). 2012.
- Ladogana A, Puopolo M, Croes EA. Mortality from Creutzfeldt-Jakob disease and related disorders in Europe, Australia, and Canada. Neurology. 2005;64(9):1586-91.
- Ladogana A, Puopolo M, Croes EA. Mortality from Creutzfeldt-Jakob disease and related disorders in Europe, Australia, and Canada. Neurology. 2005;64(9):1586-91.
- Doran M, Larner AJ. EEG findings in dementia with Lewy bodies causing diagnostic confusion with sporadic Creutzfeldt-Jakob disease. Euro J Neurology: the official journal of the European Federation of Neurological Societies. 2004;11(12):838-41.
- Manix M, Kalakoti P, Henry M. Creutzfeldt – Jakob disease: updated diagnostic criteria, treatment algorithms, and the utility of brain biopsy. Neurosurg Focus. 2015;39:E2.
- Dorey A, Tholance Y, Vighetto A. Association of cerebrospinal fluid prion protein level and distinction between Alzheimer disease and Creutzfeldt-Jakob disease. JAMA Neurol 2015;72:267-75.
- Uttley L, Carroll C, Wong R. Creutzfeldt-Jakob disease: a systematic review of global incidence, prevalence, infectivity, and intubation. Lancet Infect Dis. 2020;20:e2-e10.
- Zerr I, Hermann P. Diagnostic challenges in rapidly progressive dementia. Expert Rev Neurother. 2018;18:761-72.
- Macfarlane RG, Wroe SJ, Collinge J. Neuroimaging findings in human prion disease. J Neurol Neurosurg Psychiatry. 2007;78:664.
- Schmitz M, Ebert E, Stoeck K.. Validation of 14-3-3 protein as a marker in Sporadic Creutzfeldt-Jakob disease Diagnostic. Mol Neurobiol. 2016;53:2189-99.
- Alvarez-Buylla A, Herrera DG, Wichterle H. The subventricular zone: source of neuronal precursors for brain repair. Prog Brain Res. 2000;127:1-11.
- Belay ED. Transmissible spongiform encephalopathies in humans. Annu Rev Microbiol. 1999;53:283-314.
- Collinge J, Sidle KC, Meads J, Ironside J, Hill AF. Molecular analy- sis of prion strain variation and the aetiology of ‘new variant’. Nature. 1996;383:685-90
- Gregori L. A prototype assay to detect vCJD-infected blood. Lancet. 2011;377:444-6.
- Klingenstein R, Löber S, Kujala P, Godsave S, Leliveld SR, Gmei-P, et al. Tricyclic antidepressants, quinacrine and a novel, synthetic chimera thereof clear prions by destabi- lizing detergent-resistant membrane compartments. J Neuro Chem. 2006;98:748-59.
Article Type
Review Article
Publication History
Received Date: 10-08-2022
Accepted Date: 24-08-2022
Published Date: 31-08-2022
Copyright© 2022 by Kumar PKR. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Kumar PKR. Human CO Model of Creutzfeldt – Jakob Disease (CJD) Evaluates the Effect of Putative Drugs on Prion Accumulation. J Reg Med Biol Res. 2022;3(2):1-13.
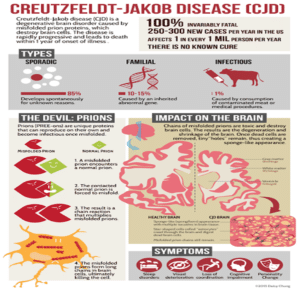
Figure 1: Above figure shows CJD and its symptoms and impact on the brain [3].
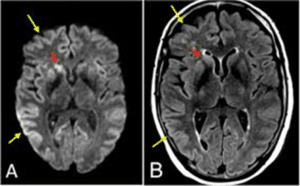
Figure 2: The scan report shows brain manifested with CJD [5].
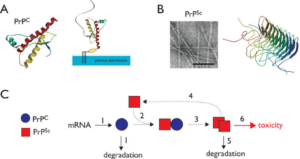
Figure 3: The above image shows prion infusion and its transmission [6].
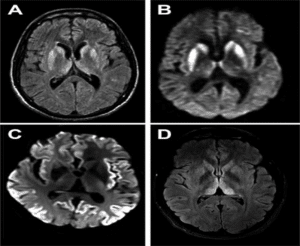
Figure 4: CJD showing sharp wave complexes [9].
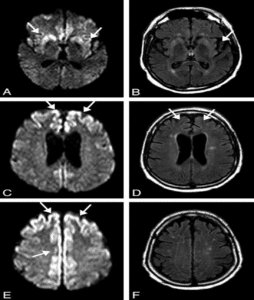
Figure 5: Pattern of cortical changes in CJD [9].
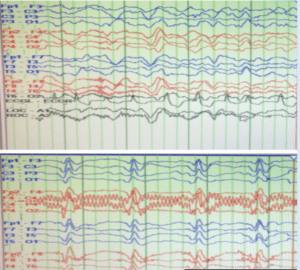
Figure 6: Electroencephalogram (EEG) demonstrates typical sCJD features described in the literature. Initially patient had periodic slow wave complexes. As his disease progressed, characteristic bilateral synchronous periodic epilentiform discharges were seen as repetitive pattern of trio basic waves approximately 1-1.5 seconds apart [10].
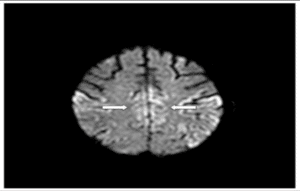
Figure 7: Hyperintense signal in cingulate gyrus.
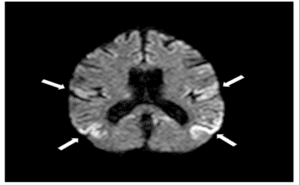
Figure 8: Restricted cortical diffusion.
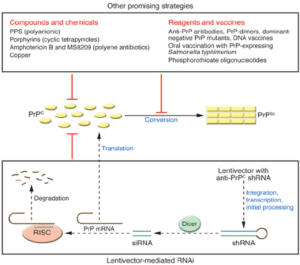
Figure 9: Promising method for prion disease cure [13].
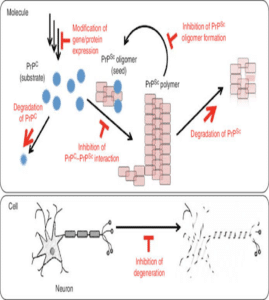
Figure 10: Inhibition of gene expression and degradation of PrP [14].
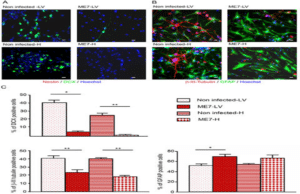
Figure 11: Prion replication in endogenous adult neural cells [15].


