Kayla Crowley1, Vincent S Gallicchio1*
1Department of Biological Sciences, College of Science, Clemson University; Clemson, South Carolina, USA
*Correspondence author: Vincent S Gallicchio, Department of Biological Sciences; 122 Long Hall, College of Science, Clemson University Clemson, South Carolina, USA; Email: [email protected]
Published Date: 29-05-2023
Copyright© 2023 by Gallicchio VS, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Tooth decay is an extremely common problem throughout the nation. As individuals pass the age of 20 years old, 90% have had some form of tooth decay. Along with this, more than 1 in 4 people have untreated tooth decay in the United States. When tooth decay is left untreated, an infection or trauma can often result in needing a root canal and many other health problems. An average of 25 million root canals are performed every year, with around 40,000 being performed each day. Root canal treatment can result in pain or infection, as well as a weakened tooth that will be lost later in life. Due to the tooth pulp not being able to regenerate, there are many research studies being carried out to find a permanent and effective treatment. Mesenchymal and dental pulp stem cells are a large interest at this time due to their wide range of abilities to regenerate dentine and pulp in a tooth with necrotic tissue. While stem cells found in oral tissue are currently being studied in-vivo as well as in-vitro, the main focus is on producing the necessary types of stem cells for full dental tissue regeneration leading to complete re-gain of function. This review outlines the findings from recent years with stem cell usage in regeneration within the oral cavity.
Keywords: Mesenchymal Stem Cells; Dental Pulp Stem Cells; Root Canal; Regenerative Endodontics
Abbreviations
MSC: Mesenchymal Stem Cells; DPSC: Dental Pulp Stem Cells; SHED: Human Exfoliated Deciduous Teeth; REP: Regenerative Endodontic Procedures; PCR: Polymerase Chain Reaction; CA9: Carbonic Anhydrase IX; sEV: Small Extracellular Vesicles
Introduction
Oral health is intricately tied with overall health and is not to be taken lightly. Tooth decay can lead to infection, which if left untreated can spread to other bodily tissues, possibly even leading to death [1]. Root canals result from a buildup of bacteria within the pulp of a tooth, causing an infected or abscessed tooth. These infected or abscessed teeth cause an inflamed pulp, which can result in pain. This is usually a result of leaving an untreated cavity, or dental decay, but can also result due to trauma injury such as a cracked tooth [2]. Tooth pain tends to spread or progress to other areas surrounding the affected tooth, including neighboring teeth and the jaw. Along with pain, a tooth requiring a root canal can have sensitivity to hot and cold temperatures, collect pus with a swollen gums and jaw, have tooth discoloration due to a lack of blood supply and can soften surrounding bone allowing for flexibility and movement with the affected tooth [2]. An affected tooth may also lead to necrosis of the cells in surrounding tissue, displaying the reverberating effects of root canal infection on other tissues as well as even organs [3].
Current Standard of Care
Root canals are often determined by the dentist through X-rays and the cavity test. Periapical X-rays are used to visualize the tooth decay and the cavity test involves drilling a small hole into the affected tooth to test the nerve tissue health [4]. Thermal and electric testing is also a well-known tactic to determine if a tooth needs a root canal. The cold test, also referred to as an Endo Ice Test, is a specific test of pulp that can help evaluate the nerve response of the tooth with intense sensitivity to cold. Cold spray is applied to a small piece of cotton, or a Q-tip and is then placed on the affected tooth for 5-10 seconds. If the cold sensitivity lasts for longer than 10 seconds after the cotton is removed from the area, then this can mean the tooth has irreversible pulpitis [5].
A root canal performed today typically takes about 30 minutes to an hour. This time can depend on the complexity of the roots, how may roots there are, the size of the tooth and the location of where it is being performed in the mouth [2]. During the procedure, the infected pulp and nerve are removed in order to clean and shape the canals. The roots and tooth are then filled and sealed, often with a crown. The crown can protect the tooth and help restore its original function as much as possible.
Problems with Root Canal Treatment
Although root canals are an efficient way to clean up decay and infection in order to help save an affected tooth, there are a lot of consequences of the procedure. Conventional root canal treatments often lead to a poor prognosis, as there is an increased risk of fracture and susceptibility to recontamination. Some of the big issues resulting in endodontic failure include persistence of bacteria within the roots, underfilling and overfilling, as well as coronal leakage [6]. An endodontically treated tooth will lack its original strength after treatment, the infection can appear again and hidden canals can cause a treatment that needs to be redone. Human error is a large factor in root canal problems and has been shown to vary depending on undergraduate schooling. When a study was done determining the technical quality of root canals of undergraduate students, the percentage of acceptable root canals done was anywhere from 10-80% [7]. Along with this, the number of unacceptable treatments drastically increased as the position of the affected tooth moved posteriorly within the oral cavity. As mentioned previously, one of the most common causes of unacceptable root canals within the study was due to underfilling or overfilling the tooth, resulting in density problems [7].
Root canal success rates are found to vary and a systemic review found success rates to be as low as 68% a year after treatment was conducted [8]. When looking at a study conducted on 411 patients with 1175 endodontically treated teeth, the prognosis of root canal therapy was determined after a 10-year period. Despite 85% of the root canals being deemed successful, 3.9% were considered only a partial success, 5.8% had either no change or were starting to worsen in condition and 4.4% were a partial failure [9]. While there was no additional information on 21 of the teeth, about 7% of the teeth ended up having to be extracted even after root canal treatment. Additional studies have found a possible tie of oral foci of infections to systemic disorders. While some researchers have struggled to find a relationship between oral sepsis and rheumatoid disease, other publications link the dental foci of infection to infective rheumatoid arthritis when studying knee joints of rabbits [3]. The microorganisms from oral infections resulting from root canals are highly capable of leading to rheumatoid arthritis.
The longevity of root canal fillings was addressed with direct and indirect dental restorations done in England and Wales. Over 80,000 patients from this study who were at least 18 years old were examined, with data being addressed using the modified Kaplan-Meier statistical analysis over an 11-year period. Root canal treated teeth were found to have a significantly shorter lifespan compared to non-root canal treated teeth, no matter the structure of the tooth (Fig. 1) [10]. These teeth had the same course of treatment and the log-rank tests found the results to be statistically significant. There were 5,557 incisors and canines that had root canal fillings, 7,965 premolars and 8.912 molar teeth [10]. The biggest differences between no root filling and root filling survival rates were seen between premolar teeth 10 years after treatment, molar teeth after 5 years and molar teeth after 10 years. Premolar teeth 10 years after treatment had a difference of 15%, molar teeth after 5 years had a difference of 15% and molar teeth after 10 years had difference of 16% (Table 1) [10]. Root canals are found to have less time since placement until intervention.
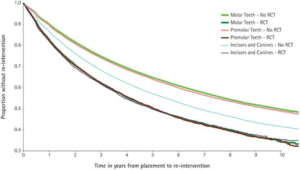
Figure 1: Survival rate of direct dental restorations with and without root canal treatment [10].
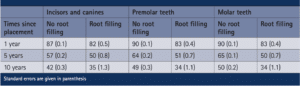
Table 1: Survival rate percentage of teeth with no root filling versus root fillings in various tooth types over time of placement [10].
Why Stem Cells are Being Investigated?
Once dental caries starts to cause damage within teeth and even lead to pulp necrosis, the damaged tissue must be removed and the tooth strength is greatly compromised. Stem cells are characteristic of having the possibility of functional regeneration of damaged dental pulp tissue, or the dentine-pulp complex [11]. The aim of using such cells is to replicate and replace the original pulp structure as well as function instead of using treatment such as fillings or root canals. Dental pulp is found to have a high ability to regenerate since it is a leader in repairing periodontal tissue when damage occurs. When such damage occurs, dental pulp cells multiply, travel to the site of damage and differentiate into odontoblasts to attempt to repair the dentin [12]. While regenerative endodontic procedures, also referred to as REPs, are treatment procedures that are designed to replace and repair damaged structures within the pulp-dentin complex, mesenchymal stem cells have been found to promote healing of damaged tissues, continued root development and growth and even hold the possibility of vitality responses [11]. Mesenchymal Stem Cells (MSCs) have been isolated from the pulp tissue of permanent teeth, resulting in the locating of Dental Pulp Stem Cells (DPSCs) and Stem Cells from Human Exfoliated Deciduous Teeth (SHED). These isolated intracanal cells were found to co-express MSC markers and demonstrate a great mineralizing differential potential [11].
Discussion
A clinic study was run with 20 adult patients with an age range from 20 to 85 years old, referred due to their need for endodontic treatment of mature teeth with apical lesions. This study was evaluating the ability of MSC to invade the root canal system when bleeding was evoked on the periapical surface of mature teeth. The goal was to address the initiation and capability of MSC reproducing the original pulp-dentine complex structure and function. Stem cells have been found to be within dental pulp, the apical papilla and the inflamed periapical tissue being examined within this study [13]. The damaged periapical tissue was removed from participants and the intracanal bleeding was completed for sampling by PCR reverse transcription using MSC-specific arrays [11]. The data was analyzed using the Wilcoxon signed-rank test. Along with PCR reverse transcription, MSCs were analyzed from the blood sample with flow cytometry and quantitative osteogenesis assay to determine the presence and distribution of stem cells within the lacerated periapical tissue. While MSC markers were found to increase in root canal systems of immature teeth in other studies, this study wanted to test the same ideas within mature teeth [14]. Since the apical papilla is known to shrink in mature dentition, other sources of initializing MSC inflow are needed. The utilized MSC markers were CD73, CD90, CD105, CD146 and CD45. MSC markers CD73, CD90, CD105 and CD146 were found to be significantly upregulated within the mature teeth lesions once the periapical tissue was removed. The median fold change values were 2.9, 31.7, 4.6 and 6.8, respectively (Fig. 2) [11]. On the other hand, CD45, a negative MSC marker was downregulated with a median fold change of -2.7. About 40% of participants had a significant upregulation in all four of the positive MSC markers, as well as a significant downregulation in the negative MSC marker [11].
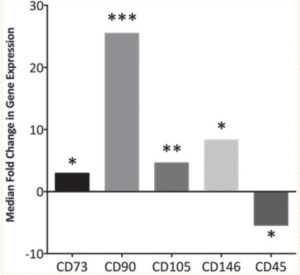
Figure 2: Fold changes in gene expression for the mesenchymal stem cell markers CD73, CD90, CD105, CD146 and CD45 found in intracanal blood samples [11].
Mesenchymal stem cells taken from the intracanal blood of participants were found to have significant mineralizing differentiation potential. The presence and abundance of MSC relative to the area of concern is directly tied to the ability for tissue-engineering [15]. Osteogenic media had significantly higher mineralization activity compared to the basal media control, showcased with the level of absorbance (Fig. 3) [11]. This study was the first to demonstrate that evoked bleeding within the periapical tissue can deliver undifferentiated MSCs to the intracanal systems within mature dentition [11]. This allows for a promising future for the possible regeneration of the pulp-dentine complex within mature teeth.
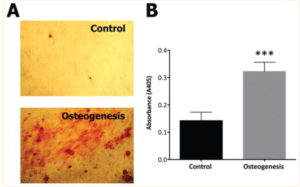
Figure 3: Results from mesenchymal stem cells cultured from intracanal sources for 2 weeks in basal or osteogenic media [11].
One of the main benefits of using stem cells is seen in their secretion of factors that enhance vascularization within the pulp, as well as providing nutrients and oxygen [16]. A study from 2011 focused on the revitalization of necrotic tissue using different stem cells. Two types of stem cells that come from dental pulp include DPSC and SHED cells. While they come from the same area of the oral cavity, SHED cells present higher rates of proliferation, alkaline phosphate activity, as well as osteocalcin production compared to DPSC [17]. Stem cells like SHED and DPSC have the ability to be multipotent, but their presence is small and the location is not extremely well known, but is now seen in perivascular niches [18]. Such stem cells can undergo odontogenic, angiogenic, adipogenic, chondrogenic, neurogenic or myogenic differentiation, which is beneficial in work with dentine-pulp complex regeneration. Since odontoblasts, or ectomesenchyme derived cells, are the first cells to respond to injury within the pulp due to bacterial invasion, when they die stem cells respond accordingly. Odontoblasts are typically induced to secrete a dentine matrix to mineralize as dentine within shallow caries, but as damage deepens to the pulp, they lose their ability or die [16]. These stem cells that respond to odontoblast death differentiate into odontoblasts once they migrate to the injury site and help with healing the damaged pulp. A main focus of this research is finding a scaffold for stem cells to be engineered into a dental pulp-like tissue. An injectable scaffold called Puramatrix was filled with SHED cells and used to create tissue the length of a root canal and was able to form new dentine along the walls (Fig. 4) [16]. Stem cells are evidently critical for response to injury within the oral cavity and there is strong potential for therapeutic targets with reversible pulpitis. This study shows the possibility of treatment of dental conditions that currently have a lack in treatability.

Figure 4: An engineered dental pulp-like tissue of human tooth generated by transplantation of SHED [16].
A major challenge that researchers have ran into with tooth regeneration is with stem cells necessary for repair. It has been discussed that two types of stem cells are necessary for proper tooth development as well as possible regeneration: oral epithelial cells and mesenchymal stem cells. While the epithelial cells are important for forming ameloblasts, the mesenchymal stem cells are more focused on differentiated cells such as pulp, ligaments and odontoblasts [19]. A huge advancement within this area of research would be finding one cell type that may differentiate into the other type, or to induce formation. Current research at Harvard School of Dental Medicine is being led by Dr. Yingzi Yang and Dr. Jennifer regarding the stem cell’s role in tooth regeneration and repair. While looking into biologically based tooth repair, both doctors plan to identify the DPSCs necessary specifically for dentin repair in molar teeth. An interesting portion of this study focuses on the effect of dental injury on the expression of the Sonic hedgehog (Shh) protein [20]. Sensory neurons are predicted to be regulators of dental stem cells as well as signaling within the damaged pulp. This research will be monumental in the goal of optimizing tooth retention as well as limiting damage to mature dentition. Due to advances in stem cell-mediated regeneration, bioactive compounds are now being studied to determine their assistance in dental regeneration with proliferation, differentiation and survival of cells. An overview of 139 studies on oral stem cell regeneration in dental, bone, neural and other tissues has had some major findings. When looking at a specific two in-vivo studies, one pre-clinical study and one in vitro study used to examine DPSCs with dental regeneration, stem cells were found to mineralize into a similar cell to odontoblasts when placed into human root canals [21]. When DPSCs were introduced to fibrin gel implants, they were able to create architecture of similar tissue vascularization to bone, but with an increased volume. DPSCs were also found to increase gene expression levels in certain mediums, including osteogenic, fibrogenic, cementogenic media. Fibrogenic genes influenced include FSP-1, PLAP-1, COL1, COL3, osteogenic genes influenced include ALP, COL1, OPN and RUNX2 and cementogenic genes influenced include BSP, CEMP1 and CAP [21]. One of the studies done in-vivo to test DPSC ability in dental pulp regeneration found stem cells to differentiate into odontoblast-like mineralizing cells and human CD31, which is promising for regenerating tissue. A following study using hPDLSCs with scaffolding in-vivo to look at bone regeneration found new bone formation to have normal vascularization and structure (Table 2) [21].
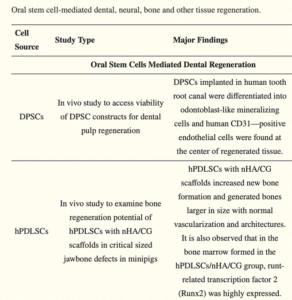
Table 2: Major findings from two studies using dental pulp stem cells [21].
Another problem that researchers are running into with DPSCs is their likelihood to undergo apoptosis with the environment and conditions are not ideal. A study looking into the use of taxifolin, which is a natural anti-hypoxic and anti-inflammatory, to protect DPSCs and reduce apoptosis in vitro. When DPSCs are exposed to conditions that are abnormal, or not ideal, 10 mM of taxifolin is able to significantly lessen the amount of apoptosis [22]. Taxifolin is also found to increase the expression of carbonic anhydrase IX, while not increasing HIF1a expression, which is very beneficial. CA9, or carbonic anhydrase IX, inhibition is counterproductive to the protective properties of taxifolin, so the increase in expression of CA9 is supportive to the protection of DPSCs from apoptosis [22].
Mesenchymal cells are also being investigated for their ability to accelerate wound healing. Mesenchymal stem cells are found to secrete Interleukin-1 Receptor Antagonist (IL-1RA), which is connected to the rapid wound healing properties in gingiva [23]. It utilizes the Fas, Fas-associated phosphatase-1 (Fap-1) and Caveolin-1 (Cav-1) cascade with small Extracellular Vesicles (sEV). Fas was found to control the release of IL-1RA-sEV in mesenchymal stem cells, as it is bound to Fap-1 and Cav-1 [23]. The complex of Fas, Fap-1 and Cav-1 regulates apoptosis, while IL-1RA-sEV is able to up-regulate the complex expression in the mesenchymal stem cells.
Studies have also been done focusing on DPSCs specific physiological function and application in regenerative medicine. When the entire dental pulp tissue is lost due to trauma, infection, injury, or decay, the regeneration necessary is de novo creation of pulp [24]. This pulp needs to be functional, including vascularization and forming within the root canal space. DPSCs require scaffolding, a growth factor and the stem cells necessary for differentiated odontoblasts. Although future studies are needed, DPSCs propose a possible way to be taken from immature teeth removed or lost by children in order to help with future regeneration, such as regenerative endodontic treatment of permanent teeth after root development. A study that looks specifically at permanent incisors with apical periodontitis attempts to revascularize pulp in the entire root canals of mature necrotic teeth. Root canals were instrumented to the apices with step-back technique and irrigated with antimicrobial solution to remove bacteria, with intracanal medicaments used inside root canal (calcium hydroxide and ciprofloxacin) [25]. Bleeding was then induced at the next appointment with hand files pushed past the apices and collagen membranes were placed in canals. This is one way modified regenerative endodontic procedures are being tested for mature dentition, but future studies are needed.
Conclusion
Mesenchymal stem cells hold a promising future with usage for dentine and pulp regeneration. As demonstrated, isolated intracanal cells that had the necrotic tissue removed from where the apical lesion was expressed an increase in MSC markers and downregulated negative MSC markers. There was no correlation with MSC expression and age, sex, tooth type or treatment type. MSC demonstrate robust mineralizing differential potential and are found to be compartmentalized mainly in vasculature. MSCs can be delivered into the root canal system in mature teeth with apical lesions when bleeding is evoked, which is a promising step in the regeneration of damaged tissue pulp within root canals. DPSCs may be the future of dentistry and although there are many challenges to come, future research will hopefully only brighten that path.
As for future research, there is a lack of studies on the possibility of the clinical delivery of MSCs to the root canal system of these mature teeth with necrotic tissue. Studies on MSC entrance into the apical surface of a tooth need a broader participant group, as current studies only have about 20 and mean age of 44 years old. While age might be a factor that does not determine amount of inflow of MSCs into the intracanal system, differentiation capacity may be affected. In addition to this, the studies focused on in this review were 45% mandibular molars, so there needs to be more studies on other areas, such as maxillary premolars and mandibular premolars. Underlying pulp angiogenic responses (formation and differentiation of blood vessels) is important for new therapies for conservation of dental pulp viability. Stem cells are a significant area of research for the dentistry and the capabilities of such cells appear to be vast. As more research and studies are completed, the possibilities of incorporating stem cell usage into the dental field becomes more likely.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- Centers for Disease Control and Prevention. Cavities. 2021. [Last accessed on May 22, 2023]
https://www.cdc.gov/oralhealth/fast-facts/cavities/index.html - Root canal: What is it, diagnosis, treatment, side effects and recovery. Cleveland Clinic. (n.d.). 2023. [Last accessed on May 22, 2023]
https://my.clevelandclinic.org/health/treatments/21759-root-canal
- Murray CA, Saunders WP. Root canal treatment and general health: a review of the literature. Int Endodontic J. 2000;33(1):1-8.
- Kishen, A. Diagnosis in endodontics: Patients at the Faculty of Dentistry Clinics (University of Toronto). Diagnosis in Endodontics | Patients at the Faculty of Dentistry Clinics (University of Toronto). 2019. [Last accessed on May 23, 2023]
https://patients.dentistry.utoronto.ca/diagnosis-in-endodontics
- Castillo-Silva BE, Alegría-Torres JA, Martínez-Castañón GA, Medina-Solís CE, Zavala-Alonso NV, et al. Diagnostic accuracy of three placement sites for the cold test in subjects amongst different age groups. BMC Oral Health. 2019;19:1-9.
- Tabassum S, Khan, FR. Failure of endodontic treatment: The usual suspects. Euro J Dent. 2016;10(01):144-7.
- Ribeiro DM, Réus JC, Felippe WT, Pachêco‐Pereira C, Dutra KL, Santos JN, et al. Technical quality of root canal treatment performed by undergraduate students using hand instrumentation: a meta‐analysis. Int Endodontic J. 2018 Mar;51(3):269-83.
- Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. Outcome of primary root canal treatment: systematic review of the literature-part 1. Effects of study characteristics on probability of success. Int Endodontic J. 2007;40(12):921-39.
- Fonzar FE, Kalemaj Z, Fabian Fonzar R, Buti J, Buttolo P, Forner Navarro L, et al. The prognosis of root canal therapy: a 20-year follow-up ambispective cohort study on 411 patients with 1169 endodontically treated teeth. Clinical Trials in Dentistry. 2021;3(02).
- Dunne S. Summary of: Influence of root canal fillings on longevity of direct and indirect restorations placed within the General Dental Services in England and Wales. British Dental J. 2014;216(6):358-9.
- Chrepa V, Henry MA, Daniel BJ, Diogenes A. Delivery of apical mesenchymal stem cells into root canals of mature teeth. J Dental Res. 2015;94(12):1653-9.
- Kwack KH, Lee HW. Clinical potential of dental pulp stem cells in pulp regeneration: current endodontic progress and future perspectives. Frontiers in Cell and Developmental Biol. 2022:734.
- Hargreaves KM, Diogenes A, Teixeira FB. Treatment options: biological basis of regenerative endodontic procedures. Pediatric dentistry. 2013;35(2):129-40.
- Lovelace TW, Henry MA, Hargreaves KM, Diogenes A. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endodontics. 2011;37(2):133-8.
- Pei M. Faculty opinions recommendation of Mesenchymal Stem Cells: Environmentally responsive therapeutics for regenerative medicine. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature. 2014.
- Rosa V, Botero TM, Nör JE. Regenerative endodontics in light of the stem cell paradigm. Int Dental J. 2011;61:23-8.
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sci. 2003;100(10):5807-12.
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone and Mineral Res. 2003;18(4):696-704.
- Angelova A, Pang Y, Sharpe PT. Stem cell-based biological tooth repair and regeneration. Trends in Cell Biology. 2010;20(12):715-22.
- Investigating the role of stem cells in tooth repair. Harvard School of Dental Medicine. 2022. [Last accessed on May 22, 2023]
https://hsdm.harvard.edu/news/researchers-investigate-role-stem-cells-repairing-teeth
- Ahuja A, Tyagi PK, Kumar M, Sharma N, Prakash S, Chandran D, et al. Botanicals and oral stem cell mediated regeneration: a paradigm shift from artificial to biological replacement. Cells. 2022;11(18):2792.
- Fu X, Feng Y, Shao B, Zhang Y. Taxifolin protects dental pulp stem cells under hypoxia and inflammation conditions. Cell Transplantation. 2021;30:09636897211034452.
- Kou X, Xu X, Chen C, Sanmillan ML, Cai T, Zhou Y, et al. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Science Translational Med. 2018;10(432):eaai8524.
- Tatullo M, Marrelli M, Shakesheff KM, White LJ. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Engineering Reg Med. 2015;9(11):1205-16.
- Paryani K, Kim SG. Regenerative endodontic treatment of permanent teeth after completion of root development: a report of 2 cases. J Endodontics. 2013;39(7):929-34.
Article Type
Review Article
Publication History
Received Date: 02-05-2023
Accepted Date: 22-05-2023
Published Date: 29-05-2023
Copyright© 2023 by Gallicchio VS, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Gallicchio VS, et al Potential Mesenchymal and Dental Pulp Stem Cell Usage for Regeneration of Dentine and Its Complications. J Reg Med Biol Res. 2023;4(2):1-9.

Figure 1: Survival rate of direct dental restorations with and without root canal treatment [10].

Figure 2: Fold changes in gene expression for the mesenchymal stem cell markers CD73, CD90, CD105, CD146 and CD45 found in intracanal blood samples [11].

Figure 3: Results from mesenchymal stem cells cultured from intracanal sources for 2 weeks in basal or osteogenic media [11].

Figure 4: An engineered dental pulp-like tissue of human tooth generated by transplantation of SHED [16].

Table 1: Survival rate percentage of teeth with no root filling versus root fillings in various tooth types over time of placement [10].

Table 2: Major findings from two studies using dental pulp stem cells [21].


