Henry E Young1-3*, Frank Lochner1,4
1Dragonfly Foundation for Research & Development, Macon, GA 31210, USA
2Henry E Young PhD Regeneration Technologies LLC, Charlotte, NC 28227, USA
3Mercer University School of Medicine, Macon, GA 31207, USA
4Cougar Creek Veterinary Consultants, Spencer, TN 38585, USA
*Corresponding Author: Henry E Young PhD, Chief Science Officer, Dragonfly Foundation for Research and Development, 101 Preston Court, Suite 101, (Corporate Office), Macon, GA 31210 USA;
Email: [email protected]
Published Date: 20-03-2021
Copyright© 2021 by Young HE, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Previous isolation studies noted the presence of native undifferentiated telomerase positive stem cells within the brains of adult rats. Further segregation of the cells from the brain isolates demonstrated the presence of telomerase positive totipotent stem cells and pluripotent stem cells within the cell isolates. Characterization studies of their differentiation potential noted that both the telomerase positive totipotent stem cells and pluripotent stem cells would form neurons, ganglion cells and glial cells, as well as cells from surface ectoderm, mesodermal and endodermal embryonic germ layer lineages. Since reports from other groups noted the presence of neural stem cells within the subventricular zone of the lateral ventricles and the dentate gyrus of the hippocampus, we wanted to ascertain the locations of these native telomerase positive totipotent stem cells and pluripotent stem cells within various regions of the adult brain. The brains from adult rats were examined. Adult rats were euthanized following the guidelines of Mercer University School of Medicine’s IACUC. The rats were perfused with ELICA fixative, the brains harvested, frozen, cryosectioned and stained with antibodies diagnostic for the endogenous totipotent stem cells, i.e., carcinoembryonic antigen-cell adhesion moleculae-1 (CEA-CAM-1) and pluripotent stem cells, i.e., stage-specific embryonic antigen-4 (SSEA-4). Both CEA-CAM-1 and SSEA-4 positive stem cells were located within the gray matter of the cerebral cortex, whereas SSEA-4 positive cells were also located in the white matter. These results suggest that the totipotent stem cells and pluripotent stem cells are a resident population of stem cells within the cerebral cortex of the adult brain. Studies are ongoing to address their functional significance in the brain.
Keywords
Adult Stem Cells; Telomerase Positive; Totipotent Stem Cells; Pluripotent Stem Cells; Brain; Cerebral Cortex
Introduction
Previous isolation studies noted the presence of telomerase positive stem cells in multiple species of mammals, e.g., mice, rats, rabbits, cats, dog, sheep, goats, pigs, horses and humans, when comparative tissues were examined, e.g., smooth muscle, skeletal muscle and blood [1]. Further segregation and characterization studies noted three general categories of telomerase positive stem cells, e.g., totipotent stem cells, pluripotent stem cells and germ layer lineage stem cells. These three categories could be distinguished based on size, trypan blue staining pattern, a unique cell surface marker, propagation in culture and differentiation potential [2].
The smallest cell, 0.1 to 2.0 microns in size, was totally trypan blue positive and stained positive for carcinoembryonic antigen-cell adhesion molecule-1 (CEA-CAM-1). The cell’s default state in culture was quiescence in the absence of a differentiation inhibitor, such as Leukemia Inhibitory Factor (LIF) or Anti Differentiation Factor (ADF). Utilizing a proliferation inducing agent, such as Platelet-Derived Growth Factor-BB (PDGF-BB), this small cell would double every 12-14 hours and would form multiple confluent layers in culture on a type-I collagen substratum. Utilizing chemical inducing agents, human recombinant proteins and exosomes derived from downstream telomerase positive stem cells, telomerase negative progenitor stem cells and differentiated cells and these small cells could be induced to form larger downstream telomerase positive stem cells, telomerase negative progenitor stem cells and differentiated cells from ectodermal, mesodermal and endodermal germ layer lineages, spermatogonia and the nucleus pulposus of the intervertebral disc, the only adult derivative of the notochord (i.e., the primary inducer of the embryo). This telomerase positive stem cell was termed a totipotent stem cell based on its ability to form all cells of the body including the gametes [2].
The intermediate-sized cells were 2.0 to 10.0 microns in size, had a variable trypan blue staining pattern and stained positive for Stage-Specific Embryonic Antigen-4 (SSEA-4). The smallest of these cells were 2.0 to <4.0 microns. They had a halo of trypan blue staining around their periphery with a center void of trypan blue staining. The next larger cell, 4.0 to <6.0 microns, had a crown of trypan blue along one side of the cell with the remainder of the cell being trypan blue negative. The cells that were 6.0 to 8.0 microns in size and >8.0 to <10 microns in size were completely devoid of trypan blue staining. Their default state in culture was also quiescence, even in the absence of LIF or ADF. Using PDGF-BB to induce proliferation, they would also form multiple confluent layers in culture on a type-I collagen substratum. Utilizing chemical inducing agents, human recombinant proteins and exosomes derived from downstream telomerase positive stem cells, telomerase negative progenitor stem cells and differentiated cells, these variable-sized cells could be induced to form larger telomerase positive stem cells, telomerase negative progenitor stem cells and differentiated cells from ectodermal, mesodermal and endodermal germ layer lineages. They would not dedifferentiate to the more primitive telomerase positive totipotent stem cell. And due to its inability to form gametes or the nucleus pulposus, these telomerase positive cells were termed as pluripotent stem cells [2].
The largest cells were 10-12 microns in size and were completely devoid of trypan blue staining but stained for Thy-1. Their default state in culture was quiescence, even in the absence of LIF or ADF. Utilizing PDGF-BB to induce proliferation, these cells would become contact inhibited, but would not die, like their progenitor stem cell cousins. Rather, upon replating, even from a single cell, they would proliferate until they reached contact inhibition and then they would stop dividing. This scenario of plating, propagation to contact inhibition and replating could be repeated multiple times without loss of differentiation potential of the cells. Utilizing chemical inducing agents, human recombinant proteins and exosomes derived from progenitor stem cells and differentiated cells, these larger telomerase positive cells could be induced to form telomerase negative progenitor stem cells and differentiated cells. None of these telomerase positive stem cells would dedifferentiate to the more primitive telomerase positive pluripotent stem cells or totipotent stem cells. In addition, there appeared to be three separate and distinct stem cell subgroups in this category [3].
One subgroup would only form cells of the surface ectoderm and neural ectoderm germ layer lineages, e.g., keratinocytes, pyramidal neurons, dopaminergic neurons, interneurons, astrocytes, oligodendrocytes and radial glial cells. They would not form cells from either the mesodermal or endodermal germ layer lineages. This subgroup was termed telomerase positive ectodermal stem cells [1,3].
A second subgroup would only form cells of the mesodermal germ layer lineage, e.g., skeletal muscle, smooth muscle, cardiac muscle, white fat, brown fat, hyaline cartilage, elastic cartilage, fibrocartilage, articular cartilage, growth plate cartilage, endochondral bone, intramembranous bone, loose fibrous connective tissue, dense fibrous connective tissue, dermis, tendon, ligament, capsules, trabeculae, scar tissue, endothelium of arteries, veins, capillaries, sinusoids and lymph vessels, red blood cells, white blood cells, platelets, kidney cells, spleen cells, etc. They would not form any cell type of ectodermal or endodermal germ layer lineages. The cells were originally termed as pluripotent mesenchymal stem cells [4]. But since there was confusion as to the capabilities of telomerase negative progenitor mesenchymal stem cells [5,6] versus these telomerase positive pluripotent mesenchymal stem cells, the terminology was changed to denote them as telomerase positive mesodermal stem cells [1-3], due to their ability to form all cells of the embryonic mesodermal germ layer lineage.
The third subgroup would only form cells of the endodermal embryonic germ layer lineage, e.g., lining cells of the lung; lining cells of the gastrointestinal system; liver oval cells, hepatocytes, biliary cells; and pancreas exocrine cells and pancreatic endocrine cells, e.g., glucagon-secreting α-cells, insulin-secreting β-cells and somatostatin-secreting d-cells. They would not form cells from either the ectodermal or mesodermal germ layer lineages. These cells were termed as telomerase positive endodermal stem cells [1,3].
We cloned telomerase positive totipotent stem cells, pluripotent stem cells, ectodermal stem cells, mesodermal stem cells, endodermal stem cells and telomerase negative mesenchymal stem cells using repetitive single cell clonogenic analysis for use as our “gold standards” (a “gold standard” for each of the cloned stem cell categories, e.g., totipotent, pluripotent, ectodermal, mesodermal, endodermal and mesenchymal) for comparative purposes for future experiments [2,3]. We then undertook isolating telomerase positive stem cells from tissues other than smooth muscle, skeletal muscle and blood with different techniques and compared them with cells isolated with our cloned “gold standards”. Representative examples of tissues and organs examined thus far are bone marrow, adipose tissue, dermis, heart, lung, kidney, skeletal muscle, spleen, brain, meninges, spinal cord, mixed motor/sensory nerves, blood vessels, organs of gastrointestinal system, endocrine organs and organs of the genito-urinary system [1]. While telomerase positive totipotent stem cells and pluripotent stem cells were present in every tissue examined, presumably due to their ability to form all cell types of the body, the telomerase positive germ layer lineage ectodermal stem cells, mesodermal stem cells and endodermal stem cells were only present in tissues and organs having their respective adult differentiated cell types. What we have seen thus far is that telomerase positive totipotent stem cells, pluripotent stem cells, ectodermal stem cells, mesodermal stem cells and endodermal stem cells and the telomerase negative mesenchymal stem cells from their respective source tissue(s) display the same characteristics of size, trypan blue staining, unique cell surface markers, propagation in culture and differentiation potential as the single cell clones as our gold standards.
As an adjunct to those studies, we began a series of experiments to determine the location of the telomerase positive cells, especially the totipotent stem cells and pluripotent stem cells, within the tissues. To spatially visualize the location of these cells we chose to use frozen cryosectioned tissue combined with our ELICA immunocytochemical assay system. We have since reported the spatial location of these cells in the skeletal muscle, bone marrow, lung, dermis, adipose tissue, kidney, heart and spleen [6-13].
The current series of studies address their location in various regions of the adult brain. Based on previous observations in the scientific literature that there was little to no regeneration of damaged neural tissue within the cerebral cortex, we postulated the following hypothesis [14]. Telomerase positive totipotent stem cells and pluripotent stem cells are not present in the adult rat cerebral cortex. Much to our surprise, this proved to be the null hypothesis. Both CEA-CAM-1 positive cells, indicative of totipotent stem cells and SSEA-4 positive cells, indicative of pluripotent stem cells, are present in the cerebral cortex in the adult rat brain.
Material and Methods
The use of animals in this study complied with the guidelines of Mercer University Institutional Animal Care and Use Committee (IACUC) and criteria of the National Research Council for the humane care of laboratory animals as outlined in the Guide for the Care and Use of Laboratory Animals, prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (National Academy Press, 1996).
Isolation of Rat Tissues
Sprague-Dawley rats, 200-250 gram, were obtained from Harlen Sprague-Dawley (Madison, WI). The rats were housed in shoebox cages with cellulose bedding. Animals were maintained on a twelve-hour light/dark cycle and had access to food and water ad libitum. The animals were acclimated to these conditions for at least one-week prior to tissue harvest.
All tissue harvesting procedures were carried out using aseptic techniques in a surgical suite dedicated for in-vivo animal experimentation. All instruments and surgical fields were sterilized by autoclave. The animals were anesthetized with 50 mg/kg sodium pentobarbital. The initial incision site, the ventral chest wall, was shaved and prepared swabbed with 10% Betadine solution. The Betadine solution was applied to the ventral chest wall and allowed to dry. Sterile field (drapes) were applied. The surgeon wore sterile gloves and a surgical mask to limit potential contamination.
A midline thoracic/abdominal incision through the anterior musculature was made from the base of the neck to the pelvis. Surgical scissors were used to spit the sternum vertically from xyphoid process to sternal notch and the bisected rib cage separated with a pediatric rib spreader. An incision was made in the pericardial sac and the heart externalized. An 18G needle was inserted into the ascending aorta, held in place with a hemostat and an incision was made in the apex of the left ventricle. Dulbecco’s phosphate buffered saline (DPBS, GIBCO, Grand Island Biological Company, Grand Island, NY) was perfused through the body using a six-foot pressure head. When fluid flowing from the apex of the left ventricle was water-clear, the perfusate was switched to ELICA fixative (4% v/v glutaraldehyde (Sigma), 2% w/v paraformaldehyde (Sigma) and 5% w/v D-glucose (Sigma) in phosphate buffered saline (PBS), osmolarity 1.0 at pH 7.4) and allowed to flow through the rat for 20 minutes to fix all organs and tissues in the body, including the brain. The ELICA fixative was used specifically to preserve antigenic sites during fixation that may have been disrupted if only 10% v/v (of 37%) aqueous formaldehyde was used for fixation.
Cryosectioning
The brain was removed from the skull, bisected along the midsagittal plane, cut coronally to derive four quadrants on each side of the midsagittal plane and rinsed with PBS (Fig. 1 and 2). The brain pieces were flash frozen and stored in liquid nitrogen (AirGas, Macon, GA) to prevent ice crystal formation with subsequent destruction of morphology of the tissues. Pieces of brain were removed, placed into OTC embedding medium (Tissue Tek OCT Compound 4583, Miles Laboratory, Ames Division, Elkhart, IN) and frozen at -20oC. The OCT/frozen brains were sectioned with a Tissue Tek Cryostat II (GMI, Ramsey, MN) to a thickness of seven microns, placed on positively charged microscope slides (Mercedes Medical, Sarasota, FL) and stored at a temperature of -20oC with a desiccant. Immunocytochemical staining was performed following established procedures for ELICA analyses [7,12,13].
Immunocytochemistry
All immunocytochemical staining procedures were carried out at ambient temperature (22°C). Seven-micron tissue sections on positively charged glass slides were removed from -20°C freezer, allowed to warm to ambient temperature, incubated in 95% alcohol to remove the OTC embedding medium and then washed in running water for five minutes. The tissue sections were incubated in 5.0% (w/v) sodium azide (Sigma, St. Louis, MO) in Dulbecco’s Phosphate Buffered Saline (DPBS, GIBCO, Grand Island, NY) for 60 minutes and washed in running water for five minutes. They were incubated in 30% hydrogen peroxide (Sigma, St. Louis, MO) for 60 minutes to irreversibly inhibit endogenous peroxidases [7]. Tissue sections were rinsed with running water for five minutes and incubated for 60 minutes with blocking agent (Vecstatin ABC Reagent Kit, Vector Laboratories Inc., Burlingame, CA) in PBS [7]. The blocking agent was removed. The sections were rinsed with running water for five minutes and incubated with primary antibody for 60 minutes. The primary antibodies consisted of 0.005% (v/v) carcinoembryonic antigen cell adhesion molecule-1 (CEA-CAM-1, clone 5.4) (D. Hixson, Department of Internal Medicine, Brown University, Providence, RI) in DPBS for totipotent stem cells, MAB-813 directed against stage-specific embryonic antigen-4 (SSEA-4) (Developmental Studies Hybridoma Bank, DSHB, Iowa City, IA) and smooth muscle alpha-actin (IA4, Sigma) in PBS for smooth muscle cells, as a positive procedural control [1,2,7]. The primary antibody was removed. The sections were rinsed with running water for five minutes and incubated with secondary antibody for 60 minutes. The secondary antibody consisted of 0.005% (v/v) biotinylated anti-mouse IgG (H + L) affinity purified, rat adsorbed (BA-2001, Vector Laboratories, Burlingame, CA) in PBS [7]. The secondary antibody was removed. The sections were rinsed with running water for five minutes and then incubated with avidin-HRP for 60 minutes. The avidin-HRP consisted of 10 ml of 0.1% (v/v) Tween-20 (ChemPure) containing 20 microliters (2 drops) of reagent-A and 20 microliters (2 drops) of reagent-B (Peroxidase Standard PK-4000 Vecstatin ABC Reagent Kit, Vector Laboratories) in PBS. The avidin-HRP was removed. The sections were rinsed with running water for five minutes and incubated with 3, 3’-diaminobenzadine (DAB) substrate (Vector) for 60 minutes. The DAB substrate consisted of 5 ml of distilled water, 40 microliters (4 drops) of DAB stock solution, 20 microliters (2 drops) of hydrogen peroxide solution and 20 microliters (2 drops) of Nickel solution (SK-4100, DAB Substrate Kit for Peroxidase, Vector). The substrate solution was removed. The sections were rinsed with running water for 10 minutes and then cover-slipped with Aqua-mount (Vector) [7].
Representative tissue sections of cardiac muscle and cerebral cortex (this study) were included to verify positive and negative procedural controls, which were included to assure validity of the ELICA immunocytochemical staining [7,12,13]. The CEA-CAM-1 positive staining control consisted of myocardium (Fig. 3) stained with antibody to CEA-CAM-1 utilizing the entire immunocytochemical procedure outlined above. The SSEA-4 positive control consisted of epicardium and underlying myocardium stained with antibody to SSEA-4 (Fig. 3) utilizing the entire immunocytochemical procedure outlined above. The IA4 positive controls consisted of myocardium (Fig. 3) and quadrant-2, level-2 cerebral cortex (Fig. 4) stained with antibody to IA4 utilizing the entire immunocytochemical procedure outlined above [12]. The negative controls consisted of the staining protocol with PBS alone without primary antibodies (CEA-CAM-1, SSEA-4, or IA4), cerebral cortex (Fig. 4); or with primary antibodies, but without secondary antibody (biotinylated anti-mouse IgG); or with primary antibodies and secondary antibody, but without avidin-HRP; or with primary antibodies, secondary antibody and avidin-HRP, but without substrate (Fig. 3) [12]. In all permutations examined, staining was absent in the negative controls.

Figure 1: Dorsal view of rat brain transected along mid-sagittal plane (A-B) and cut transversely (C, D, E) to generate eight quadrants of tissue. Left 2nd quadrant was used for this study. Photograph from Bszm.elte.hu/anatomy/mammals/67/ Atlas of Animal Anatomy and Histology, Mammals (Mammalia) Dorsal view of the brain of the rat.

Figure 2: Second quadrant (Level 2, above) prepared for sectioning, with subsequent immunocytochemical staining. Reprinted with permission from https://ntp.niehs.nih.gov/nnl/nervous/brain/index.htm

Figure 3: Seven-micron sectioned cardiac muscle used to verify primary antibody staining for CEA-CAM-1 and SSEA-4; positive procedural controls (IA4); and negative procedural controls. Reprinted with permission from Young HE, Limnios IJ, Lochner F, et al. Cardiovascular disease and adult healing cells: From bench top to bedside. J Stem Cell Res 2017; 1(3) 002:1-8 [12]. A: Positive procedural staining control for CEA-CAM-1. Myocardium of the heart. Cells stained with antibody to CEA-CAM-1, indicative of totipotent stem cells. Note red-stained clusters of cells and single cell (arrows). B: Positive procedural staining control for SSEA-4. Epicardium of heart overlying outer myocardium of heart. Cells stained with antibody to SSEA-4, indicative of pluripotent stem cells. Note red-stained cells along pericardium, surrounding a coronary blood vessel and within the outer layer of myocardium (arrows). C: Positive procedural staining control for IA4. Myocardium of heart. Tunica media of coronary blood vessel wall stained with antibody to IA4, indicative of smooth muscle alpha-actin (red stain) in wall of blood vessel. D: Negative procedural control without primary antibodies. Myocardium of heart. Used to determine non-specific binding of reagents to sectioned tissue. As shown, there was no non-specific binding of biotin, avidin-HRP, residual HRP, or substrate present in the tissue.

Figure 4: Seven micron sectioned adult rat cerebral cortex. Location of tissue from quadrant-2 (Fig. 1), level-2 (Fig. 2) of cerebral cortex. A: Stained with antibody for stage-specific embryonic antigen-4 (SSEA-4) for pluripotent stem cells (arrows) in the interface between white matter and gray matter of the cerebral cortex. Original Magnification, 800x. B: Stained with antibody for carcinoembryonic antigen-cell adhesion molecule-1 (CEA-CAM-1) for totipotent stem cells (arrows) in the interface between the gray matter and white matter of the cerebral cortex. Original magnification, 1600x. C: Stained with antibody for smooth muscle alpha-actin (IA4) (arrows) in sulci near gray matter of cerebral cortex, as a positive procedural control. Original magnification, 400x. D: Absent primary antibody, but incubated with secondary antibody and tertiary avidin-HRP probe, prior to incubation with DAB substrate. Absence of staining within gray matter of cerebral cortex denotes absence of non-specific binding of reagents to tissue and denotes the negative procedural control. Original magnification, 200x.

Figure 5: Note presence of genomically-Lac-Z-labeled cells (beta-galactosidase – brown insoluble substrate) in cerebral cortex. A: White matter containing labeled glial cells and endothelial-labeled capillary. B and C: Gray matter containing pyramidal neurons and interneurons. Reprinted with permission from Young HE, Hyer L, Black Jr AC, et al. Treating Parkinson disease with adult stem cells. J Neurol Dis 2013; 2:1 [51].
Visual Analysis
Stained sections were visualized using a Nikon TMS phase contrast microscope with bright field microscopy at 40x, 100x and 200x. Photographs were taken with a Nikon CoolPix 995 digital camera with 2x-8x electronic zoom. Digital photographs were cropped using Adobe Photoshop 7.0.
Results
The current study addresses the location of totipotent stem cells (CEA-CAM-1 positive cells) and pluripotent stem cells (SSEA-4 positive cells) in the cerebral cortex of the adult brain. Based on previous observations in the scientific literature we postulated the following hypothesis. Telomerase positive totipotent stem cells and pluripotent stem cells are not present in the adult rat cerebral cortex. This proved to be the null hypothesis. Both CEA-CAM-1 positive cells, indicative of totipotent stem cells and SSEA-4 positive cells, indicative of pluripotent stem cells, are present in the cerebral cortex in the adult rat brain. CEA-CAM-1 positive cells were located in both gray matter and white matter (Fig. 4), with a greater proportion of the cells in the gray matter than the white matter (Table 1). SSEA-4 positive stem cells were located in both the gray matter and white matter (Fig. 4) in approximately equal numbers (Table 1).
Description | Grey Matter | White Matter |
CEA-CAM-1positive Cells and Clusters | 205 | 85 |
SSEA-4 positive Cells and Clusters | 252 | 248 |
Table 1: CEA-CAM-1+ Cells and SSEA-4+ Cells per unit area of Gray Matter and White Matter of Cerebral Cortex.
Discussion
The concept of repairing and/or regenerating neurons and their networks in the central nervous system in mammals has plagued mankind for centuries [14]. Recent identification of endogenous neural stem cells and their persistent production throughout life in the adult suggests a previously unrecognized capability for self-repair [15]. Neural Stem Cells (NSCs) are a multipotent progenitor stem cell of the ectodermal germ layer lineage that has self-renewal capabilities as well as ability to form neurons, astrocytes and oligodendrocytes in the adult brain [16]. In contrast, the microglia are macrophages resident in the brain and are of the mesodermal germ layer lineage [2]. Formation of new neurons throughout the life of the individual through the proliferation and differentiation of neuronal progenitor stem cells has been reported in the subventricular zone of the lateral ventricles and in the sub-granular zone of the dentate gyrus in the hippocampus [17-22]. Only dormant progenitor neural stem cells exist in the adult cerebral cortex [23]. During in utero development, multipotent progenitor neural stem cells undergo differentiation producing a myriad of projecting neuronal cell types [24]. While the adult mammalian brain retains the capacity for neurogenesis, it diminishes with aging, casting doubt on its feasibility for therapeutic cell replacement in stroke and neurodegenerative disorders, which disproportionately affect the aged brain [25]. While ischemia-induced neurogenesis occurs in the aged brain, measures designed to augment reduced neurogenesis might have therapeutic applications [25].
Neurogenesis, activated by ischemic insult, has been shown in the subventricular zone of the lateral ventricles, in the dentate gyrus of the hippocampus and in the dormant neural stem cells in the cerebral cortex [17,23,26]. Current concepts in brain plasticity, enabling lifelong learning, suggest that there can be spontaneous recovery and that rehabilitative training may help modify and boost the neuronal plasticity processes [27,28]. There is great plasticity of the neural progenitors and the roles that both intrinsic factors and extrinsic factors have concerning their eventual cell fate. Potential therapeutic applications for these factors include local environmental cues, e.g., glycogen, Brain-Derived Neurotrophic Factor (BDNF), Rho kinase; endothelial progenitor cells; exogenous factors, e.g., clopidrogel but not aspirin; enforced physical training, rehabilitative training; and local neural activity, [27-37].
Brain trauma is a major health problem worldwide. Currently, there is no effective treatment. Recent evidence suggests that adult neural stem cells from subventricular region of lateral ventricles and the dentate gyrus of the hippocampus may play regenerative and reparative roles in response to CNS injury [38]. Cell-based transplantation therapies are viewed as an alternative option to regenerate and repair brain damage [39]. Such cells could be cultured and propagated neural stem cells isolated from the subventricular zone of lateral ventricles and the sub-granular zone of the dentate gyrus, embryonic human brain cells, embryonic rodent brain cells, immortalized progenitor cells, bone marrow-derived cells and post-mitotic neurons from human teratocarcinoma cells and have been studied in various model systems of brain damage [38-43]. The model systems studied included integration into neuronal circuits, modification of local microenvironments, local trophic support and protection of regenerating neuronal cells [41-43]. Transplantation of bone morrow-derived mesenchymal stem cells and progenitor neural stem cells have demonstrated problems of graft versus host disease response and tumorgenicity of implanted cells, suggesting that other avenues should be considered [44]. An alternative approach to transplantation of exogenous cells for replacing lost neurons due to brain injury would be to reprogram reactive glial cells into functional neurons using direct lineage programming in vivo [45-47].
A large proportion of the cell proliferation and differentiation studies using endogenous neural stem cells or exogenous cells from other sources have utilized bromodeoxyuridine to incorporate into dividing cells for regeneration and/or repair studies of the injured brain. Bromodeoxyuridine (BrdU) is a thiamine analog that incorporates into DNA of dividing cells during the S-phase of the cell cycle. It is used to monitor cell proliferation and has been instrumental in the study of neurogenesis in the adult nervous system. BrdU-labeling has been used in conjunction with differentiation of cells displaying phenotypic markers for mature neurons and genomic labeling of cells using viral vectors. The cells labeled with BrdU could be neural stem cells, endothelial cells, or other cell types undergoing proliferation and differentiation into neuronal cell types. However, in many instances, appropriate controls have been overlooked and events reported as generation of new neurons from neural stem cells have been misinterpreted, which makes BrdU labeling one of the most misused techniques in neuroscience [48-50].
The results reported herein describe two additional populations of stem cells, located in the cerebral cortex, with the potential to form neurons, astrocytes and oligodendrocytes given the appropriate local environmental cues, e.g., telomerase positive totipotent stem cells and telomerase positive pluripotent stem cells [1-3]. Indeed, when a genomically (Lac-Z) labeled undifferentiated naïve telomerase positive pluripotent stem cell clone (Scl-40β) was injected through the cerebral cortices of adult rats into the substantia nigra of the midbrain, these genomically-labeled cloned stem cells migrated to damaged areas within the cerebral cortex and, responding to local microenvironmental cues, formed neurons and glial cells, e.g., pyramidal neurons and interneurons in the gray matter and glial cells and capillary endothelial cells (mesodermal origin) in the white matter (Fig. 6) [51]. This suggested that exogenously delivered telomerase positive stem cells with the potential to form neuronal cell types could be provided to regenerate and/or repair damaged cerebral cortex with a sustained nutrient supply following injury [52].
Conclusion
Previous isolation studies noted that telomerase positive stem cells were present in the brains of adult rats. Further segregation and characterization studies noted four populations of telomerase positive stem cells, e.g., totipotent stem cells, pluripotent stem cells, ectodermal stem cells and mesodermal stem cells. These studies utilized the entire brain to isolate the stem cells. Therefore, we had no way of knowing the location of the stem cells with respect to the different regions of the brain. Previous groups had noted that multipotent progenitor neural stem cells with the capability to form neurons, astrocytes and oligodendrocytes were present in the subventricular zone of the lateral ventricles and in the dentate gyrus of the hippocampus throughout the lifetime of the individual. Additional studies noted that there was continued neurogenesis in these regions, even with aging, that might contribute to plasticity seen after trauma to the brain. Studies using BrdU in conjunction with differentiation studies of cells expressing neuronal phenotypes and genomic labeling studies suggested that neural stem cells from the subventricular zone and the dentate gyrus were the only endogenous stem cells present that could contribute to neuronal repair in the cerebral cortex. Based on those observations we formulated the following hypothesis: Telomerase positive totipotent stem cells and pluripotent stem cells are not present in the adult rat cerebral cortex. Much to our surprise, this proved to be the null hypothesis. Both CEA-CAM-1 positive cells, indicative of totipotent stem cells and SSEA-4 positive cells, indicative of pluripotent stem cells, are present in both the gray matter and white matter of the cerebral cortex in the adult rat brain. Our own extensive characterization studies, with chemical compounds, human recombinant proteins and exosomes derived from adult differentiated cells, noted that the telomerase positive totipotent stem cells and pluripotent stem cells would also form neuronal cells, e.g., neurons, dopaminergic neurons, pyramidal neurons, ganglion cells, radial glial cells, interneurons, astrocytes, oligodendrocytes and Schwann cells. This suggested that since these telomerase positive stem cells are present in the cerebral cortex, they might assist the telomerase negative neural progenitor stem cells for the plasticity seen in the cerebral cortex following injury to the adult post-natal brain.
Acknowledgements
The authors would like thank Gypsy FL Black, Julie A Collins/Coleman/Warren, Kristina C Hawkins, Caroline Alena, Vidit Krishna, Nicholas L Henson, Carrie Sidwell, Shirley Powell, Asa C. Black Jr (Mercer University School of Medicine), Jee-In Yoon (Wesleyan College, Macon, GA; College of Medicine, Ewha Woman’s University, Seoul 158-710, South Korea), Marie Carriero, Douglas Hixson (Brown University) and Cecile Duplaa (INSERM, France) for their technical assistance. These studies were funded in part by MedCen Foundation, Rubye Ryle Smith Charitable Trust, LM and HO Young Estate Trust and Dragonfly Foundation for Research and Development.
References
- Young HE, Black AC. Pluripotent stem cells, endogenous versus reprogrammed- a review. MOJ Orthop Rheumatol. 2014;1(4):00019.
- Young HE, Speight MO. Characterization of endogenous telomerase-positive stem cells for regenerative medicine- a review. Stem Cell Regen Med. 2020;4(2):1-14.
- Young HE, Speight MO. Donor selection is a critical component using naïve endogenous adult stem cells for the treatment of chronic diseases and traumatic injuries. J Regen Med & Biol Res. 2020;1(2):1-28.
- Young HE, Duplaa C, Young TM, Floyd JA, Reeves ML, Davis KH, et al. Clonogenic analysis reveals reserve stem cells in postnatal mammals-I Pluripotent mesenchymal stem cells. Anat Rec. 2001;263:350-60.
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641-50.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineages potential of adult human mesenchymal stem cells. Sci. 1999;284(5411):143-7.
- Young HE, Henson NL, Black GF, Hawkins KC, Coleman JA, Black Jr AC. Location and characterization of totipotent stem cells and pluripotent stem cells in the skeletal muscle of the adult rat. J Stem Cell Res. 2017;1(1):1-17.
- Young HE, Henson NL, Black GF, Hawkins KC, Coleman JA, Black Jr AC. Stage-specific embryonic antigen-4-positive cells and carcinoembryonic antigen cell adhesion molecule-1-positive cells are located in the bone marrow of the adult rat. J Stem Cell Res. 2017;1(2)001:1-3.
- Young HE, Black GF, Coleman JA, Hawkins KC, Black Jr AC. Pulmonary diseases and adult healing cells: from bench top to bedside. J Stem Cell Res. 2017;1(2)003:1-9.
- Young HE, Limnios JI, Lochner F, McCommon G, Black GF, Coleman JA, et al. Healing cells in the dermis and adipose tissue of the adult pig. J Stem Cell Res. 2017;1(2)004:1-5.
- Young HE, Black GF, Coleman JA, Hawkins KC, Williams S, Black Jr AC. Healing cells in the kidney of the adult rat. J Stem Cell Res. 2017;1(3)001:1-4.
- Young HE, Limnios IJ, Lochner F, McCommon G, Black GF, Coleman JA, et al. Cardiovascular disease and adult healing cells: From bench top to bedside. J Stem Cell Res. 2017;1(3)002:1-8.
- Young HE, Limnios IJ, Lochner F, McCommon G. Telomerase-positive stem cells in adult porcine and adult rat spleen. I. Totipotent stem cells. J Regen Med & Biol Res. 2020;1(2):1-20.
- Tomassy GS, Lodato S, Trayes-Gibson Z, Arlotta P. Development and regeneration of projection neuron subtypes of the cerebral cortex. Sci Prog. 2010; 93(2):151-69.
- Lichtenwalner RJ, Parent JM. Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab. 2006;26(1):1-20.
- Okan H. Stem cell biology of the central nervous system. J Neurosci Res. 2002;69(6):698-707.
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult after focal cerebral ischemia. Neuroscience. 2001;105(1):33-41.
- Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscien. 2003;9(4):261-7.
- Kernie SG, Parent JM. Forebrain neurogenesis after focal ischemic and traumatic brain injury. Neurobiol Dis. 2010;37(2):267-74.
- Landgren H, Curtis MA. Locating and labeling neural stem cells in the brain. J Cell Physiol. 2011;226(1):1-7.
- Mongiat LA, Schinder A. Adult neurogenesis and the plasticity of the dentate gyrus network. Eur J Neurosci. 2011;33(6):1055-61.
- Zhao X, Van Praag H. Steps towards standardized quantification of adult neurogenesis. Nat Commun. 2020;11(1):4275.
- Kuge A, Takemura S, Kokubo Y, Sato S, Goto K, Kayama T. Temporal profile of neurogenesis in the subventricular zone, dentate gyrus and cerebral cortex following transient focal cerebral ischemia. Neurol Res. 2009;31(9):969-76.
- Tomassy GS, Lodato S, Trayes-Gibson Z, Arlotta P. Development and regeneration of projection neuron subtypes of the cerebral cortex. Sci Prog. 2010;93(2):151-69.
- Jin K, Minami M, Xie L, Sun Y, Mao XO, Wang Y, et al. Ischemia-induced neurogenesis is preserved but reduced in the aged rodent brain. Aging Cell. 2004;3(6):373-7.
- Lichtenwalner RJ, Parent JM. Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab. 2006;26(1):1-20.
- Hara Y. Brain plasticity and rehabilitation in stroke patients. J Nippon Med Sch. 2015;82(1):4-13.
- Gu Y, Janoschka S, Ge S. Neurogenesis and hippocampal plasticity in adult brain. Curr Top Behav Neurosci. 2013;15:31-48.
- Tomassy GS, Lodato S, Trayes-Gibson Z, Arlotta P. Development and regeneration of projection neuron subtypes of the cerebral cortex. Sci Prog. 2010;93(2):151-69.
- Toda T, Gage FH. Review: adult neurogenesis contributes to hippocampal plasticity. Cell Tiss Res. 2018;373(3):693-709.
- Ikrar T, Guo N, He K, Besnard A, Levinson S, Hill A, et al. Adult neurogenesis modifies excitability of the dentate gyrus. Front Neural Circuits. 2013;7:204.
- Wang LL, Li J, Gu X, Wei L, Yu SP. Delayed treatment of 6-Bromoindirubin-3’-oxime stimulates neurogenesis and functional recovery after focal ischemic stroke in mice. Int J Dev Neurosci. 2017;57:77-84.
- Miyamoto N, Maki T, Shindo A, Liang AC, Maeda M, Egawa N, et al. Astrocytes promote oligodendrogenesis after white matter damage via brain-derived neurotrophic factor. J Neurosci. 2015;35(14):14002-8.
- Ding J, Li QY, Yu JZ, Wang X, Sun CH, Lu CZ, et al. Fasudil, a Rho kinse inhibitor, drives mobilization of adult neural stem cells after hypoxia/reoxygenation injury in mice. Mol Cell Neurosci. 2010;43(2):201-8.
- Fan Y, Shen F, Frenzel T, Zhu W, Ye J, Liu J, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 2010;67(4):488-97.
- Hwang M, Park HH, Choi H, Lee KY, Lee YJ, Koh SH. Effects of aspirin and clopidrogel on neural stem cells. Cell Biol Toxicol. 2018;34(3):219-32.
- Lee SH, Kim YH, Kim YJ, Yoon BW. Enforced physical training promotes neurogenesis in the subgranular zone after focal cerebral ischemia. J Neurol Sci. 2008;269(1-2):54-61.
- Rolfe A, Sun D, Kobeissy FH. Stem cell therapy in brain trauma: implications for repair and regeneration of injured brain in experimental TBI models. In: Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis. 2015:42.
- Shimogawa T, Sakaguchi H, Kikuchi T. Therapeutic effects of combined cell transplantation and locomotor training in rats with brain injury. NPJ Regen Med. 2019;4:13.
- Sun D. The potential of endogenous neurogenesis for brain repair and regeneration following traumatic brain injury. Neural Regen Res. 2014;9(7):688-92.
- Maegele M, Schafer U. Stem cell-based cellular replacement strategies following Traumatic Brain Injury (TBI). Minim Invasive Ther Allied Technol. 2008;17(2):119-31.
- Longhi L, Zanier ER, Royo N, Stocchetti N, McIntosh TK. Stem cell transplantation as a therapeutic strategy for traumatic brain injury. Transpl Immunol. 2005;5(2):143-8.
- Cao Q, Benton RL, Whittemore SR. Stem cell repair of central nervous system injury. J Neurosci Res. 2002;68(5):501-0.
- Kase Y, Shimazaki T, Okano H. Current understanding of adult neurogenesis in the mammalian brain: how does adult neurogenesis decrease with age? Inflamm Regen. 2020;40:10.
- Torper O, Gotz M. Brain repair from intrinsic sources: turning reactive glia into neurons. Prog Brain Res. 2017;230:69-97.
- Vignoles R, Lentini C, d’Orange M, Heinrich C. Direct lineage reprogramming for brain repair: breakthroughs and challenges. Trends Mol Med. 2019;5(10):897-914.
- Heinrich C, Spagnoli FM, Beringer B. In-vivo reprogramming for tissue repair. Nat Cell Biol. 2015;17(3):204-11.
- Taupin P. BrdU immunocytochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53(1):198-214.
- Landgren H, Curtis MA. Locating and labeling neural stem cells in the brain. J Cell Physiol. 2011;226(1):1-7.
- Surugiu R, Glavan D, Popescu M, Margaritescu O, Eugen R, Popa-Wagner A. Vascular remodeling in a rat model of cerebral ischemia. The fate of the BrdU-labeled cells prior to stroke. Front Neurol. 2018;27; 9:1014.
- Young HE, Hyer L, Black Jr AC, Robinson Jr JS. Adult stem cells: from bench-top to bedside. In: Tissue Regeneration: Where Nanostructure Meets Biology 3DBiotech, North Brunswick, NJ 2013;1:1-60.
- Young HE, Duplaa C, Katz R, Thompson T, Hawkins KC, Boev AN, et al. Adult-derived stem cells and their potential for tissue repair and molecular medicine. J Cell Molec Med. 2005;9:753-69.
Article Type
Research Article
Publication History
Received Date: 15-02-2021
Accepted Date: 12-03-2021
Published Date: 20-03-2021
Copyright© 2020 by Young HE, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Young HE, et al. Telomerase Positive Totipotent Stem Cells and Pluripotent Stem Cells in the Adult Brain I. Cerebral Cortex. J Reg Med Biol Res. 2021;2(1):1-16.
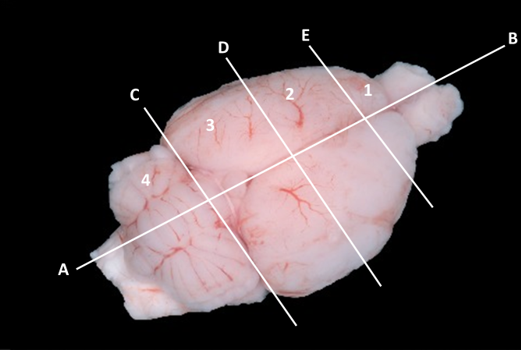
Figure 1: Dorsal view of rat brain transected along mid-sagittal plane (A-B) and cut transversely (C, D, E) to generate eight quadrants of tissue. Left 2nd quadrant was used for this study. Photograph from Bszm.elte.hu/anatomy/mammals/67/ Atlas of Animal Anatomy and Histology, Mammals (Mammalia) Dorsal view of the brain of the rat.

Figure 2: Second quadrant (Level 2, above) prepared for sectioning, with subsequent immunocytochemical staining. Reprinted with permission from https://ntp.niehs.nih.gov/nnl/nervous/brain/index.htm

Figure 3: Seven-micron sectioned cardiac muscle used to verify primary antibody staining for CEA-CAM-1 and SSEA-4; positive procedural controls (IA4); and negative procedural controls. Reprinted with permission from Young HE, Limnios IJ, Lochner F, et al. Cardiovascular disease and adult healing cells: From bench top to bedside. J Stem Cell Res 2017; 1(3) 002:1-8 [12]. A: Positive procedural staining control for CEA-CAM-1. Myocardium of the heart. Cells stained with antibody to CEA-CAM-1, indicative of totipotent stem cells. Note red-stained clusters of cells and single cell (arrows). B: Positive procedural staining control for SSEA-4. Epicardium of heart overlying outer myocardium of heart. Cells stained with antibody to SSEA-4, indicative of pluripotent stem cells. Note red-stained cells along pericardium, surrounding a coronary blood vessel and within the outer layer of myocardium (arrows). C: Positive procedural staining control for IA4. Myocardium of heart. Tunica media of coronary blood vessel wall stained with antibody to IA4, indicative of smooth muscle alpha-actin (red stain) in wall of blood vessel. D: Negative procedural control without primary antibodies. Myocardium of heart. Used to determine non-specific binding of reagents to sectioned tissue. As shown, there was no non-specific binding of biotin, avidin-HRP, residual HRP, or substrate present in the tissue.
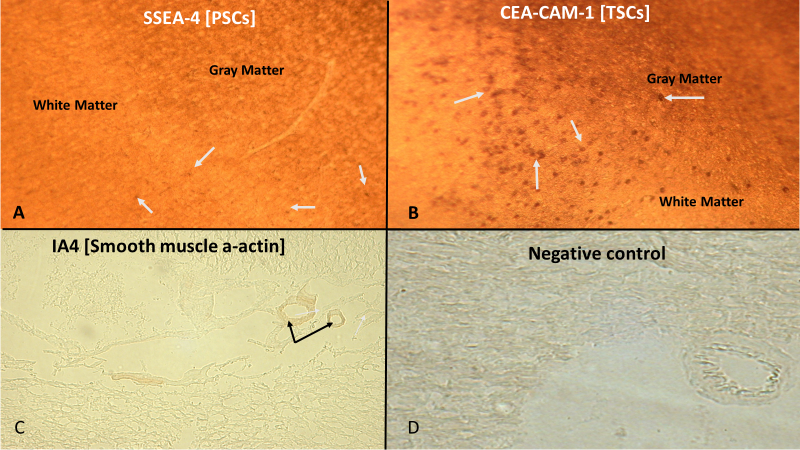
Figure 4: Seven micron sectioned adult rat cerebral cortex. Location of tissue from quadrant-2 (Fig. 1), level-2 (Fig. 2) of cerebral cortex. A: Stained with antibody for stage-specific embryonic antigen-4 (SSEA-4) for pluripotent stem cells (arrows) in the interface between white matter and gray matter of the cerebral cortex. Original Magnification, 800x. B: Stained with antibody for carcinoembryonic antigen-cell adhesion molecule-1 (CEA-CAM-1) for totipotent stem cells (arrows) in the interface between the gray matter and white matter of the cerebral cortex. Original magnification, 1600x. C: Stained with antibody for smooth muscle alpha-actin (IA4) (arrows) in sulci near gray matter of cerebral cortex, as a positive procedural control. Original magnification, 400x. D: Absent primary antibody, but incubated with secondary antibody and tertiary avidin-HRP probe, prior to incubation with DAB substrate. Absence of staining within gray matter of cerebral cortex denotes absence of non-specific binding of reagents to tissue and denotes the negative procedural control. Original magnification, 200x.

Figure 5: Note presence of genomically-Lac-Z-labeled cells (beta-galactosidase – brown insoluble substrate) in cerebral cortex. A: White matter containing labeled glial cells and endothelial-labeled capillary. B and C: Gray matter containing pyramidal neurons and interneurons. Reprinted with permission from Young HE, Hyer L, Black Jr AC, et al. Treating Parkinson disease with adult stem cells. J Neurol Dis 2013; 2:1 [51].
Description | Grey Matter | White Matter |
CEA-CAM-1positive Cells and Clusters | 205 | 85 |
SSEA-4 positive Cells and Clusters | 252 | 248 |
Table 1: CEA-CAM-1+ Cells and SSEA-4+ Cells per unit area of Gray Matter and White Matter of Cerebral Cortex.


