Home » A Case of a Patient with Combined Diabetic Peripheral Neuropathy and Peripheral Vasculopathy Who Achieved Healing After Combined Multidisciplinary Outpatient Treatment of the Diabetic Foot
Case Report | Vol. 5, Issue 1 | Journal of Clinical Medical Research | Open Access |
A Case of a Patient with Combined Diabetic Peripheral Neuropathy and Peripheral Vasculopathy Who Achieved Healing After Combined Multidisciplinary Outpatient Treatment of the Diabetic Foot
Qing Jia1



1Diabetic Foot Multidisciplinary Clinic, Huadong Hospital Affiliated to Fudan University, Shanghai City, China
2Department of Geriatrics, Huadong Hospital Affiliated to Fudan University, Shanghai City, China
*Correspondence author: Jiao-Jiao Bai, Diabetic Foot Multidisciplinary Clinic, Huadong Hospital Affiliated to Fudan University, Shanghai City, China; Email: bjj163163@163.com; Mariya.qin@hotmail.com
Citation: Jia Q, et al. A Case of a Patient with Combined Diabetic Peripheral Neuropathy and Peripheral Vasculopathy Who Achieved Healing After Combined Multidisciplinary Outpatient Treatment of the Diabetic Foot. Jour Clin Med Res. 2024;5(1):1-8. http://dx.doi.org/10.46889/JCMR.2024. 5109
Copyright© 2024 by Jia Q, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
| Received 12 Mar, 2024 | Accepted 03 Apr, 2024 | Published 10 Apr, 2024 |
Abstract
Diabetic foot ulcer is one of the serious complications of diabetes, which is a chronic wound caused by a combination of factors such as limb ischemia, infection, lower limb neuropathy, etc. Its treatment and wound management pose significant challenges to all healthcare professionals. Currently, treatment for diabetic foot ulcers often focuses on surgical interventions such as skin grafting and amputation, significantly impacting patients’ quality of life. A 61-year-old female patient presented to the clinic due to a swollen skin breakdown on the right inner ankle. She was diagnosed with diabetes at the age of 53 and developed her first diabetic foot ulcer at the age of 60. Laboratory results from this visit revealed a glycosylated hemoglobin level of 9.7%, a glycosylated albumin level of 26.8% and a fasting blood glucose level of 16.1 mmol/L. Ultrasonography of the lower limb arteries showed localized intimal-medial thickening with multiple sclerotic plaque formation on both lower limbs. For this patient, the multidisciplinary joint outpatient clinic for the diabetic foot was given systemic treatments such as blood glucose control, nerve nourishment, circulation improvement, lipid regulation and plaque fixation, while the diabetic foot care specialist gave an individualized wound treatment plan of cleansing, debridement and dressing coverage by evaluating the wound’s pH, infection grading and exudate traits, among other indicators. After 2 months of comprehensive treatment, the patient’s blood glucose control was stable and the ulcer healed. This case of recurrent ulcer rehabilitation provides new ideas for diabetic foot ulcer wound treatment and individualized wound treatment based on diabetic foot care led by experts holds promise as another effective means for healing diabetic foot ulcers.
Keywords: Diabetic Foot Ulcer; Recurrence; Ulcer Healing; The Whole Process
Abbreviations
DFU: Diabetic Foot Ulcer; DPN: Diabetic Peripheral Neuropathy; PAD: Peripheral Arterial Disease; CGMS: Continuous Glucose Monitoring System; ABI: Ankle-Brachial Index
Introduction
Diabetic Foot Ulcer (DFU) is an ischaemic, neurological and neuro-ischemic lesion of the foot caused by diabetes mellitus, which is one of the serious complications of diabetes mellitus with high recurrence and amputation rates and it is the leading cause of non-traumatic lower limb amputation worldwide [1]. The high-glucose environment in diabetic patients can lead to increased levels of oxidative stress in the body, causing local tissue damage, chronic inflammation formation and foot ulcers that are difficult to heal. Moreover, even after healing, the recurrence rate and amputation rate remain high. The risk of recurrence is 40%within 1 year and 65% within 5 years of ulcer healing [2]. Furthermore, although amputation removes local foci of infection and maintains a high level of limb function while avoiding severe bacteremia, amputation results in significant alterations to the biomechanics of the foot, such as plantar pressures and gait, which greatly increases the risk of DFU recurrence [3]. Winkley, et al., a recurrence of a full-layer epidermal breach of not less than 5 mm in diameter at the same or a different site of a previous ulcer is an ulcer recurrence [4]. Ulcer recurrence serves as a significant indicator of deteriorating health in diabetic patients, severely impacting their quality of life and imposing a substantial burden on both families and society. The cost of treating a completely healed diabetic foot in the United States can reach up to $3959 [5].
Diabetic Peripheral Neuropathy (DPN) and Peripheral Arterial Disease (PAD) are important risk factors for the development and progression of diabetic foot ulcers [6]. These two lesions increase the risk of delayed healing, non-healing and amputation of foot ulcers [7]. Connor H, et al., included only patients with diabetic foot ulcers in combination with neuropathy in their study and showed that the rate of ulcer recurrence was higher in this population as compared to other studies [8,9]. Peripheral neuropathy is present in over 10% of individuals diagnosed with type 2 diabetes, which suggests that the burden of diabetic foot disease will continue to increase in the future [10]. Patients with diabetic foot ulcers have increased the chances of foot ulcer recurrence due to neuropathy, which causes structural changes in the foot and increased plantar pressure. Peripheral Arterial Disease (PAD) represents a local manifestation of atherosclerosis in the lower limbs, affecting both microvascular and large vascular systems [11]. It often causes abnormalities in lower limb circulation, inadequate tissue perfusion, exacerbating nerve damage and delaying wound healing. In DFU patients, the prevalence of PAD is 50% [12]. Therefore, prevention is greater than cure for diabetic patients and early recognition of DPN and PAD is of great importance. We report here a case of recurrent diabetic foot ulcer. This patient had a combination of diabetic peripheral neuropathy and lower limb vasculopathy and refused surgical treatment.
We report here a case of recurrent diabetic foot ulcer. This patient had a combination of diabetic peripheral neuropathy and lower limb vasculopathy and refused surgical treatment. On this visit, she received systemic treatment in a multidisciplinary joint diabetic foot clinic and personalized ulcer rehabilitation by a diabetic foot care specialist, achieving tissue epithelialization and complete healing of the ulcer in 2 months.
Ethical Statement
Ethics Committee approval was obtained from the Institutional Ethics Committee of Fudan University Affiliated Huadong Hospital to report this case (20230022).
Case Report
Investigations
The patient was diagnosed with diabetes in June 2015 at the age of 53. Following medical advice, she has been taking metformin hydrochloride tablets orally at a dose of 0.5g/day to control blood sugar levels. On February 2, 2022, she was hospitalized for treatment of an ulcer on her right foot. During the hospitalization, a right lower limb arterial angiography was performed under intravenous anesthesia, revealing arterial sclerosis occlusion in the lower limb vessels. Subsequently, balloon angioplasty was immediately performed. Postoperatively, she received anticoagulant, blood circulation-promoting, vasodilator and blood sugar control medications, as well as wound dressing changes for the foot ulcer. The ulcer achieved healing on March 1, 2022. On August 1, 2023, at the age of 61, the patient presented to the clinic due to recurrent friction of the shoe upper against the inner ankle of the right foot, resulting in swelling, ulceration and significant pain, with the wound persisting without healing. The patient reported numbness in the limbs, cold intolerance in the lower limbs and no intermittent claudication. Following assessment by a diabetic foot care specialist, relevant examinations were promptly completed. Diabetic peripheral neuropathy examination revealed diminished ankle reflexes, decreased vibration sensation, positive 10 g nylon monofilament test, reduced sensation to temperature and coolness, decreased sensation to pinprick and weakened dorsalis pedis and posterior tibial arteries, indicating the presence of peripheral neuropathy. Lower limb vascular screening showed an Ankle-Brachial Index (ABI) of 0.8 for the left lower limb, an Ankle-Brachial Index (ABI) of 0.7 for the right lower limb and an ABI ≤ 0.90 to diagnose lower limb ischaemia. The results of the lower limb arterial ultrasound examination showed localized intimal-medial thickening with multiple sclerotic plaque formation bilaterally. Laboratory findings showed glycated hemoglobin of 9.7%, glycated albumin of 26.8% and fasting blood glucose of 16.1 mmol/L.
Diagnosis
Based on the patient’s past medical history and various examination findings, the patient was diagnosed with diabetic foot, diabetic peripheral vascular disease and diabetic distal symmetrical peripheral neuropathy.
Treatment, Follow-Up and Outcomes
Based on the patient’s examination results and his refusal to undergo surgery, the multidisciplinary outpatient clinic for diabetic foot provided systemic treatments such as glycaemic control, nerve nourishment, circulatory improvement, lipid regulation, plaque stabilization, etc. At the same time, diabetic foot care specialists assessed the pH value of the wounds, the infection grading and exudate characteristics, etc and provided individualized wound treatments such as cleansing, debridement and covering with dressings to the patient.
The patient followed medical advice to use an insulin pump (NovoRapid) for continuous subcutaneous insulin injections to control blood sugar levels, administering insulin before each of the three meals (5U before breakfast, 4U before lunch, 4U before dinner). Additionally, a Continuous Glucose Monitoring System (CGMS) was utilized to monitor blood glucose fluctuations over 72 hours. The patient orally took 50 mg of Epalrestat Tablets before each meal to treat peripheral neuropathy and alleviate adverse sensations such as pain and numbness. She also took Beraprost sodium tablets, 20µg each time, three times a day, to dilate blood vessels, increase local blood flow and prevent worsening of atherosclerosis. Furthermore, she took Levofloxacin tablets once daily, with a dose of 1 g each time, to control localized wound infection.
According to the International Wound Hygiene Concept, diabetic podiatry care specialists cleaned and debrided the wounds, removed necrotic tissue from the wounds and chose the appropriate dressing to cover the wounds according to the infection, exudate and pH level, every two days. On 1 August 2023, the day of the consultation, the first assessment of the ulcer was that it was 6.5 cm x 3.5 cm in size, the exudate was a moderate amount of plasma mixed with bloody fluid, the ulcer was markedly swollen, Wagner grade 2, there was a significant infection and the wound exudate had an alkaline pH (Fig. 1). After this wound was cleaned and debrided, the diabetic foot care specialist chose to use a wound cleansing liquid dressing (Neutro Phase), whose main ingredient is a saline solution containing pure hypochlorite, which has an extremely strong antiseptic effect, acidifies the wound environment and facilitates ulcer healing. The second assessment of the ulcer on 8 August 2023 resulted in an ulcer measuring 2 cm x 1.6 cm, with maceration present at and around the wound edges, pallor, exudate that was moderately plasmatic and an alkaline wound pH (Fig. 2). The wound was cleaned and debrided with a wound cleansing liquid dressing (Neutro Phase) to induce acidification of the ulcerated wound, while a lipid hydrocolloid dressing (Urgotul Ag/Silver) was selected to cover the ulcer, which gradually releases silver sulfate ions in contact with the wound’s exudate to control infection. It also protects the surrounding skin from adhesion to the wound and surrounding tissues. On 15 August 2023, the third assessment of the ulcer was that it was 1.8 cm x 1.5 cm in size, with a small amount of plasma exudate, the wound edges were still macerated and the wound pH was neutral (Fig. 3). Based on the assessment results, the diabetic podiatry nursing specialist, after basic wound cleansing and debridement, applied a Biatain silicone foam dressing, which has a tight fit to the wound bed, absorbs exudate vertically, reduces the risk of rupture and maceration of the surrounding skin and can reduce the pain and damage to the wound edges and the skin around the wound to a greater extent when it is removed. Since then, the patient has been coming to the outpatient clinic on a regular weekly basis for ulcer treatment and the patient’s right foot ulcer completely healed on 29 September 2023, over a period of 2 months, with significant points of ulcer healing being recorded (Fig. 4).
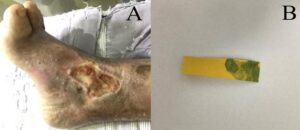
Figure 1: The condition of the foot ulcer on the day of consultation. (A): The ulcer was located above the right inner ankle, Wagner grade II, the ulcer size was 6.5 cm x 3.5 cm and the exudate was a moderate amount of plasma-blood mixture. The base of the wound consisted of 75% yellow necrotic tissue and 25% red granulation tissue with indistinct margins around the wound; (B): Measurement by pH paper showed a wound pH of 9, suggesting that the wound environment was alkaline and not conducive to wound healing.
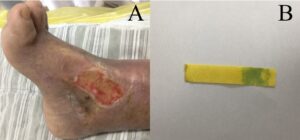
Figure 2: The second wound assessment. (A): The ulcer was located just above the right medial ankle, Wagner grade II, the ulcer measured 6.5 cm x 3.5 cm, the exudate was a moderate amount of plasma and the wound had not shrunk from the previous one, but the wound was cleaner. The base of the wound consisted of 50% red granulation tissue and 50% yellow necrotic tissue and the skin around the wound was macerated and pale. Measurement by pH paper showed that the wound pH was 8, suggesting that the wound environment was still alkaline; (B): The pH was lower than before and the wound environment tended to be better, which was favorable for wound healing.
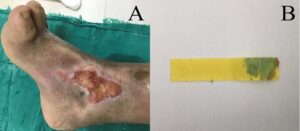
Figure 3: The third wound assessment. (A): The ulcer was located just above the right medial ankle, Wagner grade II and measured 4.8 cm x 2.1 cm with a small amount of plasma exudate. The base of the wound consisted of >75% red granulation tissue and <25% yellow necrotic tissue, with partial maceration of the periwound skin present; (B): The wound pH was 7 as measured by pH paper, suggesting that the wound environment had progressed from alkaline to acidic and the wound size was significantly smaller than before.
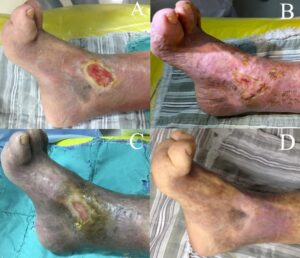
Figure 4: The patient’s entire course of outpatient follow-up to final recovery. (A): On 24 August 2023, outpatient follow-up treatment, the ulcer size was 2 cm × 3 cm; (B): on 7 September 2023, the ulcer size was 1.8 cm × 1.6 cm; (C): on 14 September 2023, the ulcer size was 0.5 cm × 0.3 cm; (D): on 29 September 2023, the ulcer was completely recovered.
Discussion
In this case, through the comprehensive treatment of a multidisciplinary joint outpatient clinic for diabetic foot, the patient was given systemic therapies such as hypoglycemia, nerve nourishment, improvement of circulation, regulation of lipids and fixation of plaque as well as individualized wound dressing change therapy, which ultimately helped the patient to achieve healing of the ulcer within 2 months.
Recurrent foot ulcers are more complex, more critical and more difficult to treat. Studies have shown that elderly diabetic patients are the main constituents of diabetic foot ulcers as well as amputations and they are more susceptible to recurrent foot ulcers due to slower metabolic rate, poorer circulation, reduced neurosensory deterioration and tissue regeneration and the combination of multiple underlying diseases [13]. Örneholm, et al., found in a 2-year follow-up that only 19% of elderly patients with recurrent foot ulcers healed after severe debridement, 4% healed after minor amputation and 5% healed after major amputation [14]. Additionally, Tabanjeh, et al., found that the 2-year recurrence rate of Diabetic Foot Ulcers (DFU) was 53.9% and lower limb deformity was a risk factor for DFU recurrence. In this case, the patient’s age of 61 years, 8-year history of diabetes, poor glycemic control, concomitant diabetic peripheral neuropathy and peripheral vascular disease and foot deformities are all risk factors for DFU recurrence and affect wound healing.
In clinical practice, special attention should be paid to elderly patients with long-standing diabetes and poor glycemic control, with a focus on strengthening post-healing medication therapy, including glycemic control and vascular treatment. It is important to emphasize that in the case of the patient reported here, the direct cause of ulcer recurrence was improper shoe selection, with repeated friction between the inner ankle of the right foot and the shoe upper, resulting in foot swelling and ulceration. Therefore, in the treatment of recurrent ulcers in the diabetic foot, healthcare professionals need to identify the causes of ulcer recurrence, provide effective health education on diabetic foot care and enhance patient self-efficacy. Patients should be empowered to play a role in treatment compliance, prevention of foot infections and avoidance of trauma, thereby preventing recurrence.
Another point to emphasize is the importance of professional wound management for ulcer healing. Regarding the treatment of diabetic foot ulcers, they are essentially typical chronic wounds. They often stagnate in the inflammatory phase for a long time due to microenvironmental changes such as alkaline wound beds, temperatures lower than the ideal healing temperature and an imbalance in humidity. Normal skin tissue pH ranges from 4 to 6, which can prevent infection by bacteria and fungi. However, when the integrity of the skin is compromised, the local pH can reach 7.4 or even as high as 8 to 9. At this point, the wound is more susceptible to bacterial colonization by organisms like Staphylococcus aureus and Pseudomonas aeruginosa, leading to protein degradation and delayed healing. Acidifying the wound environment can significantly lower the pH of wound exudate, reduce the opportunity for bacterial colonization in chronic wounds and inhibit bacterial growth.
In this case, the diabetic foot care specialist used the pH value of wound exudate as an objective indicator for auxiliary wound assessment, identifying signs of wound infection. She employed an acidic solution (Neutro Phase) to create an acidic environment in the wound, controlling wound infection and facilitating an ideal environment for wound healing. This approach improved treatment outcomes and represents a potential clinical strategy to promote wound recovery.
There have been no previous reports describing the recovery of diabetic foot ulcer patients in a combined multidisciplinary diabetic foot clinic. The diabetic patient in this case had multiple risk factors for wound healing and wound treatment was difficult. Diabetic foot care specialists carried out a full wound management practice based on assessment of wound exudate pH, infection and exudate properties and the patient eventually achieved ulcer healing. We hope that this case will provide new insights for wound management of clinical diabetic foot ulcers. However, wound treatment is complex and requires comprehensive assessment of the patient’s condition and provide individualized treatment during clinical therapy.
Conclusion
This case report demonstrates that combined multidisciplinary care is essential for the rehabilitation of diabetic foot ulcers. Additionally, wound pH-based assessment to guide wound management practices is effective for diabetic foot ulcer healing and should be another clinical strategy for future diabetic foot ulcers.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Acknowledgement
Acknowledge those who provided technical support during the study.
Financial Disclosure
This study was supported by the Fudan University Fuxing Nursing Research Fund for the “Mechanism of Health Behavior Degradation and Early Warning Strategies in Elderly Diabetic Patients with Dangerous Feet from the Perspective of Ego Depletion Theory” (No. FNF202301).
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Consent for Publication
Informed consent was obtained from the patient for publication.
Author’s Contribution
QJ and PFS contributed to the concept of this manuscript and the acquisition and evaluation of the data. QJ. drafted the manuscript. All authors provided critical revision of the manuscript and approved the final manuscript.
References
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019,157:107843.
- Hicks CW, Canner JK, Mathioudakis N, Lippincott C, Sherman RL, Abularrage CJ. Incidence and risk factors associated with ulcer recurrence among patients with diabetic foot ulcers treated in a multidisciplinary setting. J Surg Res. 2020;246:243-50.
- Fournier C, Singbo N, Morissette N, Thibeault MM. Outcomes of diabetic foot ulcers in a tertiary referral interdisciplinary clinic: a retrospective canadian study. Can J Diabetes. 2021;45(3):255-60.
- Winkley K, Stahl D, Chalder T, Edmonds ME, Ismail K. Risk factors associated with adverse outcomes in a population-based prospective cohort study of people with their first diabetic foot ulcer. J Diabetes and its Complications. 2007;21(6):341-9.
- Almobarak AO, Awadalla H, Osman M, Ahmed MH. Prevalence of diabetic foot ulceration and associated risk factors: an old and still major public health problem in Khartoum, Sudan? Ann Transl Med. 2017;5(17):340.
- Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22(7):1036-42.
- Leibson CL, Ransom JE, Olson W. Peripheral arterialdisease, diabetes and mortality. Diabetes Care. 2004;27(12):2843-9.
- Connor H, Mahdi OZ. Repetitive ulceration in neuropathic patients. Diabetes Metab Res Rev. 2004;1:S23-8.
- Tabanjeh SF, Hyassat D, Jaddou H, Younes NA, Robert AA, Ajlouni K. The frequency and risk factors of diabetic foot ulcer recurrence among jordanian patients with diabetes. Curr Diabetes Rev. 2020;16(8):910-5.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). The Lancet. 1998;352(9131):837-53.
- Jude EB, Eleftheriadou I, Tentolouris N. Peripheral arterial disease in diabetes-a review. Diabet Med. 2010;27(1):4-14.
- Stoberock K, Kaschwich M, Nicolay SS, Mahmoud N, Heidemann F, Rieß HC, et al. The interrelationship between diabetes mellitus and peripheral arterial disease. Vasa. 2021;50(5):323-30.
- Almobarak AO, Awadalla H, Osman M, Ahmed MH. Prevalence of diabetic foot ulceration and associated risk factors: an old and still major public health problem in Khartoum, Sudan? Ann Transl Med. 2017;5(17):340.
- Örneholm H, Apelqvist J, Larsson J, Eneroth M. Recurrent and other new foot ulcers after healed plantar forefoot diabetic ulcer. Wound Repair Regen. 2017;25(2):309-15.
- Tabanjeh SF, Hyassat D, daddou H. The freguency and risk factors of diabetic foot ulcer recurrence among jordanian patients with diabetes. Curr Diabetes Rev. 2020;16(8):910-5.
- Sayadi LR, Banyard DA, Ziegler ME, Obagi Z, Prussak J, Klopfer MJ, et al. Topical oxygen therapy and micro / nanobubbles: a new modality for tissue oxygen delivery. Int Wound J. 2018;15(3):363-74.
- Niaz T, Shabbir S, Noor T, Abbasi R, Imran M. Alginate-caseinate based pH-responsive nano-coacervates to combat resistant bacterial biofilms in oral cavity. Int J Biol Macromol. 2020;156:1366-80.
- Castaño O, Pérez-Amodio S, Navarro-Requena C, Mateos-Timoneda MÁ, Engel E. Instructive microenvironments in skin wound healing: Biomaterials as signal releasing platforms. Adv Drug Deliv Rev. 2018;129:95-117.
- Niaz T, Shabbir S, Noor T, Abbasi R, Imran M. Alginate-caseinate based pH-responsive nano-coacervates to combat resistant bacterial biofilms in oral cavity. Int J Biol Macromol. 2020;156:1366-80.
This work is licensed under a Creative Commons Attribution 2.0 International License.
Author Info
Qing Jia1



1Diabetic Foot Multidisciplinary Clinic, Huadong Hospital Affiliated to Fudan University, Shanghai City, China
2Department of Geriatrics, Huadong Hospital Affiliated to Fudan University, Shanghai City, China
*Correspondence author: Jiao-Jiao Bai, Diabetic Foot Multidisciplinary Clinic, Huadong Hospital Affiliated to Fudan University, Shanghai City, China; Email: bjj163163@163.com; Mariya.qin@hotmail.com
Copyright
Copyright© 2024 by Jia Q, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Citation: Jia Q, et al. A Case of a Patient with Combined Diabetic Peripheral Neuropathy and Peripheral Vasculopathy Who Achieved Healing After Combined Multidisciplinary Outpatient Treatment of the Diabetic Foot. Jour Clin Med Res. 2024;5(1):1-8. http://dx.doi.org/10.46889/JCMR.2024. 5109

