Home » Exceptional Clinical Response to Alpelisib in a Patient with Metastatic Breast Cancer with Hyperbilirubinemia and a PIK3CA Mutation
Case Report | Vol. 5, Issue 1 | Journal of Clinical Medical Research | Open Access |
Exceptional Clinical Response to Alpelisib in a Patient with Metastatic Breast Cancer with Hyperbilirubinemia and a PIK3CA Mutation
Elim Kuo1



1Department of Hematology Oncology, Orlando Health, Orlando, Florida, USA
2Division of Academic Affairs and Research, USA
*Correspondence author: SJ Carlan, MD, Division of Academic Affairs and Research, USA; Email: stevecarlan@gmail.com
Citation: Kuo E, et al. Exceptional Clinical Response to Alpelisib in a Patient with Metastatic Breast Cancer with Hyperbilirubinemia and a PIK3CA Mutation. Jour Clin Med Res. 2024;5(1):1-7. http://dx.doi.org/10.46889/JCMR.2024. 5107
Copyright© 2024 by Kuo E, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
| Received 05 Feb, 2024 | Accepted 26 Feb, 2024 | Published 04 Mar, 2024 |
Abstract
In the era of personalized and precision medicine, the importance of next-generation sequencing in metastatic breast cancer has been increasingly recognized. This case highlights one such example in which a patient with heavily pretreated, hormone receptor-positive metastatic breast cancer was discovered to have a PIK3CA mutation and started on a PIK3 inhibitor with an exceptional response. This patient had been facing a visceral crisis with diffuse liver metastasis and hyperbilirubinemia. Alpelisib was able to achieve rapid disease control within a matter of weeks with clinical and objective laboratory improvement. To date, there has been no published data regarding the safety of alpelisib in patients with elevated bilirubin and this case report lends support to safety and efficacy in this situation.
Keywords: Breast Cancer; PI3K Inhibitor; PIK3CA Mutation; Precision Medicine; Targeted Therapy
Introduction
Metastatic breast cancer remains an incurable disease, with a median overall survival of 36 months [1]. Treatment of metastatic breast cancer is dependent on tumor characteristics and molecular subtypes [2]. Seventy percent of breast cancers are hormonally driven and Estrogen Receptor or Progesterone Receptor (ER/PR) positive [3]. Human epidermal growth factor receptor 2 (HER2) positive tumors make up 15-20% of all breast cancers and triple-negative tumors comprise 15%-20% [4,5]. Hormone Receptor (HR) positive breast cancers are treated with hormone-blocking therapy, often in conjunction with the CDK4/6 inhibitors, palbociclib, ribociclib or abemaciclib, in the first- or second-line setting [3]. Following progression on hormonal agents, treatment is with single-agent chemotherapy, unless an actionable mutation is identified [2,6].
Next-Generation Sequencing (NGS) has impacted our understanding and treatment of breast cancer by identifying molecular and genetic alterations in tumors, which can be acted upon by drug therapy [2]. One such molecular alteration is the activating PIK3CA mutation, which is found in 40% of HR-positive breast cancer patients. Eighty percent of PIK3CA mutations will occur in the helical kinase domain of p110a [7]. Alpelisib is a PI3K inhibitor that selectively targets the protein p110-alpha isoform, binding 50 times more efficiently to this isoform than others [8]. Alpelisib was studied in the SOLAR-1 trial, which randomized patients with HR+, HER2- advanced breast cancer with PIK3CA mutation who previously received endocrine therapy, to alpelisib plus fulvestrant versus placebo plus fulvestrant. The study found that the alpelisib arm had improved progression-free survival, 11.0 months (95% confidence interval [CI], 7.5 to 14.5) compared to 5.7 months (95% CI, 3.7 to 7.4) in the placebo arm [3]. This led to FDA approval of alpelisib in May 2019 for this patient population.
Here we describe a 67-year-old female with ER/PR+, HER2 negative, PIK3CA mutated, heavily pre-treated, metastatic breast cancer, with diffuse liver metastasis and hyperbilirubinemia, who had dramatic improvement of hepatic metastases after starting therapy with alpelisib and fulvestrant.
Ethical Statement
The project did not meet the definition of human subject research under the purview of the IRB according to federal regulations and therefore was exempt.
Case Report
A 67-year-old woman diagnosed in 1990 with node-negative right breast cancer was treated with lumpectomy and radiation therapy. At that time, she declined adjuvant hormonal therapy and chemotherapy. In 1998, she had a recurrence in her right breast which was treated with a mastectomy. The tumor was 1 cm, ER negative, PR positive, HER-2/neu untested. She was on observation until February 2013, when imaging found two soft tissue masses in the chest wall invading into the sternum, measuring 2.6 cm and 1.5 cm. Biopsy of one of the lesions showed poorly differentiated carcinoma, ER 100%, PR 99%, HER-2/neu negative by Fluorescence In Situ Hybridization (FISH). The patient was started on letrozole in April 2013. In July 2016, a bone scan showed increased uptake in the distal sternum, so therapy was changed to fulvestrant and palbociclib. In September 2018, a Positron Emission Tomography (PET) scan showed new hypermetabolic lesions in the sternum and right iliac bone. She was treated with capecitabine until her tumor markers trended upward and PET/CT (computed tomographic) scan in December 2019 showed the progression of disease with new hypermetabolic liver lesions as well as mediastinal adenopathy. At that point, she was shifted to carboplatin and gemcitabine. The patient received 3 cycles but then had progression with diffuse liver metastasis (Fig. 1), so she was shifted to doxorubicin and cyclophosphamide (AC). The patient completed 4 cycles of AC in May 2020 with PET/CT showing excellent therapeutic response and decreasing tumor markers. She was then started on single-agent paclitaxel.
However, in May 2021, the patient had significant progression in her liver. PET/CT was unable to be obtained due to insurance, so CT imaging was ordered that showed multiple lesions throughout the liver, one lesion measuring up to 10 x 4 cm with areas of necrosis (Fig. 2). Imaging also revealed multiple new pulmonary and pleural-based nodules bilaterally and mediastinal lymphadenopathy. Her total bilirubin was 7.7 mg/dL (0.3- 1.0 mg/dL). Tumor markers included Carcinoembryonic Antigen (CEA) of 73.7 ng/mL (<3.0 ng/mL) and CA 27.29 of 316 U/mL (0-38 U/mL). The patient was experiencing abdominal pain, jaundice and profound fatigue secondary to the liver metastases. Due to the elevated bilirubin level, chemotherapy options were limited. Polymerase Chain Reaction (PCR) testing on her tumor tissue revealed a PIK3CA mutation, specifically the p.E545K mutation. In May 2021, she was started on alpelisib and fulvestrant. Alpelisib is typically dosed at 300 mg once daily, but since there were no guidelines for dosing with hyperbilirubinemia, our patient started initially with alpelisib 150 mg daily for 14 days and then increased to 300 mg daily thereafter. She had an impressive response to therapy. Her total bilirubin and tumor markers trended down within weeks. By July 2021, total bilirubin was 1.0 mg/dL, CEA 4.4 ng/mL and CA 27.29 was 33 U/mL (Fig. 3-6). PET/CT scan in November 2021, six months after initiating therapy with alpelisib, showed complete resolution of liver lesions and no evidence of disease (Fig. 3).
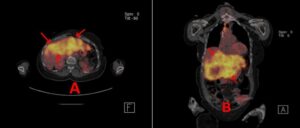
Figure 1: A and B: PET CT scan 2/26/20. Progressive disease (red arrows in liver while on carboplatin/gemzar prior to adriamycin/Cytoxan.
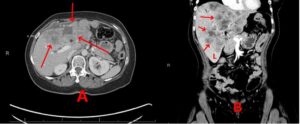
Figure 2: A and B: CT abdomen 5/12/21 showing significant progression in liver while on single agent taxol, prior to alpelisib therapy, red arrows.
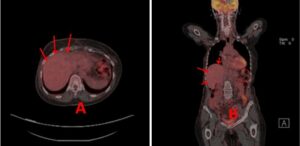
Figure 3: A and B: CT abdomen 5/12/21 showing significant progression in liver while on single agent taxol, prior to alpelisib therapy, red arrows.
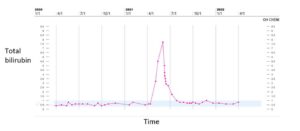
Figure 4: Total bilirubin trend.
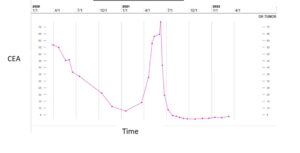
Figure 5: CEA trend.
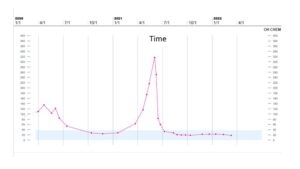
Figure 6: CA 27.29 trend.
The patient did experience hyperglycemia, a known side effect of alpelisib, requiring hospitalization about 2 weeks after initiating therapy. She had no prior history of diabetes and was found to have a blood glucose of 500 mg/dL (65-100 mg/dL) during an outpatient follow-up visit. She was hospitalized and started on an insulin drip. Despite this event, the patient wished to continue alpelisib with no dose reduction and her blood glucose was better controlled with an insulin pump. The patient showed excellent therapeutic response to alpelisib and fulvestrant for one year before progressing in her liver. She received 3 cycles of trastuzumab deruxtecan but ultimately died of liver failure. Before her passing, she consented to the publication of her genomic and clinical data.
Discussion
Alpelisib is a novel therapeutic approach that can produce excellent responses in patients with metastatic breast cancer harboring a PI3KCA mutation. Our patient with HR+, PIK3CA+ heavily pre-treated breast cancer with diffuse liver metastasis and hyperbilirubinemia had a near complete response to alpelisib and fulvestrant. She was on a full dose of alpelisib 300 mg daily as there were no guidelines to dose-adjust with elevated bilirubin. This case demonstrates that alpelisib can be safely administered with a gradual increase to the full dose in patients with elevated bilirubin without significant toxicities.
In the era of precision personalized medicine, testing for PI3K mutation should be undertaken for all patients with metastatic breast cancer. This can be undertaken through plasma or tissue sampling. When PI3K mutations are present in cancer, it is acquired as a somatic mutation [9]. A study published in 2014 found that PI3K mutation status does not change over time for most patients with breast cancer [10]. Thus, testing can occur at any point after diagnosis of HR+/HER2- advanced or metastatic breast cancer. The FDA has approved the therascreen PIK3CA Companion Diagnostic Test (CDT) from Qiagen to assess PIK3CA mutational status [9]. This test utilizes qualitative polymerase chain reaction to detect 11 mutations of the PIK3CA gene and can be run on plasma or tissue. While plasma testing is less invasive and more convenient, there is the potential for false negatives. In the SOLAR-1 trial, only 56% of patients who had tumor tissue-confirmed PIK3CA mutation had the mutation identified in the plasma specimen [11]. Thus, if the plasma testing for the mutation is negative, the practitioner should confirm with testing on the tumor tissue itself.
The most frequent grade 3 or 4 adverse events found in clinical trials were hyperglycemia (36.6% in the alpelisib-fulvestrant group vs 0.7% in the placebo-fulvestrant group) and rash (9.9% vs 0.3%) [3]. As the PI3K/mTOR signaling pathway is also involved in glucose homeostasis, hyperglycemia is an on-target effect [8]. Hyperglycemia of any grade was found in 65% of patients treated with alpelisib, with median time to onset for grade ≥2 hyperglycemia of 15 days [8]. Patients should be counseled of this potential adverse effect and have baseline fasting blood glucose and HgA1c prior to initiation of treatment. Glucose control should be optimized prior to starting therapy and monitored once a week for the first two weeks, then once every 4 weeks and as clinically indicated [11]. If the patient develops hyperglycemia, anti-hyperglycemic agents may be used and dose reductions of alpelisib can be considered.
Skin rashes are also a class effect of PI3K inhibitors; maculopapular rash, pruritus and dry skin are the most encountered adverse events [8]. Prophylactic antihistamines during initiation of alpelisib can reduce the incidence of skin reactions and treatment typically consists of topical or oral corticosteroids depending on the severity [8].
Conclusion
While metastatic breast cancer is still a largely incurable disease, targeted therapy has provided additional options for patients that have progressed on endocrine therapy and chemotherapy. In patients with a PI3K mutation, the addition of alpelisib to anti-estrogen therapy may provide efficacious and durable treatment response. This case emphasizes the importance of next generation sequencing in breast cancer and the excellent response to alpelisib despite heavy pretreatment. It also showed that full dose alpelisib can be used in hyperbilirubinemia, providing our patient approaching liver failure with limited treatment options another avenue for disease control.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Acknowledgement
Acknowledge those who provided technical support during the study.
Financial Disclosure
No funding was not involved in the manuscript writing, editing, approval or decision to publish.
Data Availability
All data generated or analyzed in this study are included in this article. Access to data is possible with permission from the responsible author.
Consent for Publication
Informed consent was obtained from the patient for publication of this case report and is stated in the manuscript.
Author’s Contribution
Writing the initial draft of the manuscript: JK, SB, EK
Conceptualization and supervision: EK, JK
Medical management of the case: EK, SB
Revising the manuscript critically and literature review: EK, JK, SC
References
- Karihtala P, Jääskeläinen A, Roininen N, Jukkola A. Prognostic factors in metastatic breast cancer: a prospective single-centre cohort study in a Finnish University Hospital. BMJ Open. 2020;10(10):e038798.
- Lang GT, Jiang YZ, Shi JX, Yang F, Li XG, Pei YC, et al. Characterization of the genomic landscape and actionable mutations in Chinese breast cancers by clinical sequencing. Nature Comm. 2020;11(1):5679.
- André F. Alpelisib for PIK3CA -mutated, hormone receptor-positive advanced breast cancer. New Eng J Med. 2019;380:1929-40.
- Piccart M, Procter M, Fumagalli D, De Azambuja E, Clark E, Ewer MS, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. J Clin Oncol. 2021;39(13):1448-57.
- Zagami P, Carey LA. Triple negative breast cancer: Pitfalls and progress. NPJ Breast Cancer. 2022;8(1):95.
- Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Epidemiology and risk factors. Breast Cancer. Lancet. 2021;397:1750-69.
- Mayer IA, Arteaga CL. PIK3CA Activating mutations: a discordant role in early versus advanced hormone-dependent estrogen receptor-positive breast cancer? J Clin Oncol. 2014;32(27):2932-4.
- Rugo HS, Lacouture ME, Goncalves MD, Masharani U, Aapro MS, O’Shaughnessy JA. A multidisciplinary approach to optimizing care of patients treated with alpelisib. The Breast. 2022;61:156-67.
- Martínez-Sáez O, Chic N, Pascual T, Adamo B, Vidal M, González-Farré B, et al. Frequency and spectrum of PIK3CA somatic mutations in breast cancer. Breast Cancer Res. 2020;22(1):1-9.
- Arthur LM, Turnbull AK, Renshaw L, Keys J, Thomas JS, Wilson TR, et al. Changes in PIK3CA mutation status are not associated with recurrence, metastatic disease or progression in endocrine-treated breast cancer. Breast Cancer Res Treat. 2014;147(1):211-9.
- Wilhoit T, Patrick JM, May MB. Alpelisib: a novel therapy for patients with PIK3CA-mutated metastatic breast cancer. J Advanced Pract Oncol. 2020;11(7):768.
Author Info
Elim Kuo1



1Department of Hematology Oncology, Orlando Health, Orlando, Florida, USA
2Division of Academic Affairs and Research, USA
*Correspondence author: SJ Carlan, MD, Division of Academic Affairs and Research, USA; Email: stevecarlan@gmail.com
Copyright
Copyright© 2024 by Kuo E, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Citation: Kuo E, et al. Exceptional Clinical Response to Alpelisib in a Patient with Metastatic Breast Cancer with Hyperbilirubinemia and a PIK3CA Mutation. Jour Clin Med Res. 2024;5(1):1-7. http://dx.doi.org/10.46889/JCMR.2024. 5107

