Home » Internal Mammary Artery Graft Flow Steal by a Large Dialysis Arteriovenous Fistula Characterized by Electrical Storm
Case Report | Vol. 5, Issue 1 | Journal of Clinical Medical Research |
Internal Mammary Artery Graft Flow Steal by a Large Dialysis Arteriovenous Fistula Characterized by Electrical Storm
Kiera Brigh Turner1





1Department of Internal Medicine, Orlando Regional Healthcare System, Orlando, Florida, USA
2Department of Internal Medicine, Wright State University Boonshoft School of Medicine, USA
3Department of Pediatrics, Wright State University Boonshoft School of Medicine, USA
4Charles E Schmidt College of Medicine, Florida Atlantic University, USA
5Delray Medical Center, Delray, Florida, USA
6Department of Academic Affairs and Research, USA
*Correspondence author: SJ Carlan, MD, Division of Academic Affairs and Research, USA; Email: stevecarlan@gmail.com
Citation: Turner KB, et al. Internal Mammary Artery Graft Flow Steal by a Large Dialysis Arteriovenous Fistula Characterized by Electrical Storm. Jour Clin Med Res. 2024;5(1):1-5. http://dx.doi.org/10.46889/JCMR.2024. 5108
Copyright© 2024 by Turner KB, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
| Received 08 Mar, 2024 | Accepted 28 Mar, 2024 | Published 06 Apr, 2024 |
Abstract
Background: Steal syndrome describes a condition in which a dilated vessel distal to a smaller artery attempts to compensate for decreased blood flow by “stealing” from the smaller artery. Steal syndrome is not uncommon and can be seen in certain physiologic and pathologic states. The classic example is an occluded atherosclerotic coronary artery resulting in a misdirection of blood flow into the open channels that have developed over time resulting in downstream ischemia.
Case Report: A 66-year-old male fourteen years post 4-vessel coronary artery bypass grafting using his left internal mammary artery to bypass a stenotic segment of the left anterior descending artery, along with three saphenous venous conduits to the right coronary artery. He also had a stent placement by PCI. He was on dual antiplatelet therapy with aspirin and clopidogrel. An electrophysiology study revealed inducible VF and he underwent implantation of a dual chamber ICD 9 months prior to presentation. He experienced an unprovoked fall at home and a workup revealed the flow volume through the patient’s fistula increased to such a degree that the patient developed a steal syndrome from his left internal mammary artery graft. In addition, went into a state of electrical storm caused by ischemic damage to cardiac tissue distal to the graft. By ligating the fistula, the patient’s steal syndrome resolved and he did not suffer further ventricular arrhythmias.
Conclusion: Both coronary bypass grafting and fistula formation are common and necessary interventions and providers should take care to monitor for interactions between these proposed interventions to best serve their patients without causing additional harm. Increased vascular flow through any portion of the body can induce a steal syndrome from an upstream region. Patients who undergo arteriovenous (AV) fistula placement may develop a steal syndrome from ipsilateral bypass grafts.
Keywords: Steal Syndrome; Coronary Artery Bypass Graft; Arteriovenous Fistula; Electrical Storm
Abbreviations
PCI: Percutaneous Intervention; VF: Ventricular Fibrillation; ICD: Implantable Cardioverter Defibrillator; AV: Arteriovenous; LIMA: Left Internal Mammary Artery; LAD: Left Anterior Descending Artery; ES: Electrical Storm; VT: Ventricular Tachycardia; CABG: Coronary Artery Bypass Graft
Introduction
Steal syndromes are a relatively common phenomenon that describes a flow dynamic present in certain physiologic and pathologic states [1]. To compensate for atherosclerosis, certain stenotic vessels become permanently dilated. Unobstructed vessels respond to stress by themselves dilating and “stealing” blood flow from those stenotic vessels, causing downstream ischemia. Coronary stress tests rely on steal phenomena to visualize potential ischemia. Steal syndrome is also seen in certain pathologic conditions such as subclavian steal, wherein the dilated segment distal to a narrowed subclavian artery attempts to compensate for increased demand by “stealing” blood flow from the ipsilateral vertebral artery. The reversal of vertebral blood flow may induce vertebrobasilar insufficiency that classically presents as “drop attacks,” in which patients experience sudden syncope related to upper extremity movements. Arteriovenous (AV) fistulas create a high-flow and low-resistance vascular system that may also instigate steal syndromes. Temporary flow reduction in the internal mammary arteries has been observed during hemodialysis using ipsilateral upper extremity AV fistulas [2]. When the Left Internal Mammary Artery (LIMA) is grafted to the Left Anterior Descending Artery (LAD), patients may experience left ventricular hypokinesis with classic angina lasting for the duration of hemodialysis from a right brachial AV fistula [2].
Myocardial ischemia is a precursor lesion for Electrical Storm (ES). ES is defined as three or more episodes of sustained Ventricular Tachycardia (VT) or Ventricular Fibrillation (VF) within twenty-four hours or two or more episodes of VT or VF within five minutes. Monomorphic ventricular tachycardia is the most common arrhythmia associated with ES [3]. ES is more common in patients who obtain an ICD for secondary prevention, occurring in 10-20% of these patients compared to 4% of those who obtain an ICD for primary prevention [4]. Low ejection fraction less than 25% or increased QRS greater than 120 ms may also predict patients at higher risk for ES [5]. ES is usually a sign of severe endocardial damage that has created malicious isthmuses through which recurrent deadly rhythms can flow [6]. ES is often treated medically with beta-blockers and antiarrhythmic therapy with amiodarone as a first-line treatment [3]. Catheter ablation can also be an effective treatment for ES, especially for patients presenting with monomorphic VT [4].
Ethical Statement
The project did not meet the definition of human subject research under the purview of the IRB according to federal regulations and therefore was exempt.
Case Report
The patient is a 66-year-old male with a past medical history of hypertension, diabetes mellitus type 2, coronary artery disease with prior Coronary Artery Bypass Grafting (CABG), Percutaneous Intervention (PCI), heart failure with reduced ejection fraction of 35-40% at baseline, Implantable Cardioverter-Defibrillator (ICD) and end-stage renal disease on dialysis, who presented after a fall. Fourteen years prior to presentation he underwent a 4-vessel CABG using his LIMA to bypass a stenotic segment of the LAD, along with three saphenous venous conduits to the right coronary, first diagonal and left circumflex arteries. Nine months prior to arrival he experienced an aborted sudden cardiac death, resulting in stent placement by PCI. He started dual antiplatelet therapy with aspirin and clopidogrel. An electrophysiology study revealed inducible VF and he underwent implantation of a dual chamber ICD.
Six months before presentation he started regular hemodialysis three days a week using a right tunneled dialysis catheter. A left upper extremity AV fistula was placed and allowed to mature. He started using the mature AV fistula for treatments one week prior to presentation. Several days before presentation he developed shortness of breath at rest, four-pillow orthopnea and paroxysmal nocturnal dyspnea. On the day of his initial presentation, he suddenly became lightheaded with rapid palpitations while walking in his home. He sat down to rest and these symptoms resolved after 15 to 30 minutes. He then stood up and was walking to the bathroom when he fell. He denied loss of consciousness, but he also stated that he did not remember falling. He suffered a laceration to his lower lip with no other injuries. He presented to a local hospital where computed tomography demonstrated a right subdural hematoma. He was transferred to a nearby level one trauma center. Aside from his lip injury, he was asymptomatic.
Labs revealed hypokalemia with potassium 3.1 mEq/L (reference range: 3.5 to 5.1 mEq/L) and hypomagnesemia at 1.6 mg/dL (reference range: 1.8 to 2.5 mg/dL). His creatinine was 5.0 mg/dL (reference range: 0.6 to 1.2 mg/dL) and his BUN was 70 mg/dL (reference range: 8 to 26 mg/dL). An electrocardiogram showed sinus tachycardia at 104 bpm with frequent premature ventricular complexes and fusion complexes. An echocardiogram revealed left ventricular ejection fraction of 15-20% with anterior and lateral wall motion abnormalities. Repeat head computed tomography showed anterior and posterior falx subdural hematomas measuring up to 3 mm each.
Initial management included repair of his laceration and repletion of potassium and magnesium. His ICD was interrogated revealing 3 episodes of polymorphic VT requiring shocks in the previous 24 hours, one of which coincided with his fall. Meeting the criteria for ES, he was started on continuous intravenous amiodarone at 0.5 mg/min. Cardiac catheterization was deferred as his subdural hematomas required holding his antiplatelet therapy.
Overnight his condition worsened and he experienced 13 episodes of recurrent polymorphic VT requiring ICD shocks. He was started on continuous intravenous lidocaine at 1 mg/min. Despite the risks associated with his hematomas, his daily aspirin 81 mg was restarted to allow for urgent cardiac catheterization.
Coronary angiography and intravascular ultrasound revealed underexpansion and severe in-stent restenosis of the left main, left circumflex and ramus intermedius. Balloon angioplasty was performed with no additional stent placement. These branches, however, were of very small caliber and diffusely diseased. Saphenous venous conduits to the first diagonal and right coronary arteries were found to be occluded, while the saphenous venous conduit to the first left posterolateral branch was patent. Angiography of the left subclavian artery revealed very brisk flow with faint filling of the LIMA. The LIMA was patent on selective angiography, with subtle retrograde flow to the subclavian artery noted at the ostium (Fig. 1).
Given the findings of coronary angiography and the drop in left ventricular ejection and wall motion abnormalities, there was concern for subclavian steal syndrome, with an excessive arterial flow through the left subclavian and AV fistula leading to “steal” from the LIMA. Vascular surgery was consulted and performed duplex ultrasound, which revealed a widely patent left upper extremity AV fistula with flow volume estimated at an extraordinarily high rate of 6,075 mL/min (reference range: 600-1,500 mL/min) [4,7]. He underwent ligation of the left AV fistula. Following ligation, patient was successfully weaned off antiarrhythmic therapy. Telemetry revealed frequent premature ventricular complexes and occasional short runs of non-sustained VT that did not require ICD shocks. He had symptomatically improved and experienced no further syncope. He was discharged home with plans to obtain a right upper extremity AV fistula as an outpatient.
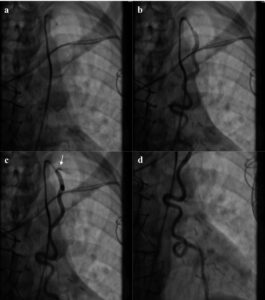
Figure 1: Coronary Steal Syndrome from the patent LIMA graft. Left heart catheterization images in chronological order of (a-b,d) a patent LIMA graft with; (c) retrograde flow from the LIMA to the subclavian (white arrow).
Discussion
This case illustrates an example of steal syndrome from a CABG caused by a high flow ipsilateral left upper extremity AV fistula. Myocardial ischemia caused by iatrogenic steal syndrome related to this high-output AV fistula was likely a strong contributing factor to this patient’s ES.
The most likely etiology for this patient’s ES is ischemic damage secondary to the visualized steal syndrome. The acute reduction in left ventricular ejection fraction by echocardiography in addition to anterior and lateral wall motion abnormalities suggest ischemic damage related to the LAD. Cardiac catheterization demonstrated a patent LIMA to LAD bypass graft with brisk subclavian flow and proximal retrograde flow to the subclavian artery (Fig. 1). Moreover, consistent with his presentation, coronary steal is likely exacerbated during hemodialysis sessions [2]. High AV fistula flow may also contribute to subendocardial ischemia due to supply-demand mismatch.
The timing of his symptom onset and resolution also suggests ischemia from steal syndrome as the etiology of his ES. His AV fistula’s maturation for use with dialysis therapy coincided with the onset of his symptoms of shortness of breath and orthopnea and his ES resolved once regular flow was restored by AV fistula ligation. Other potential etiologies of this patient’s ES include electrolyte abnormalities or a malfunctioning pacemaker [8]. These causes are less likely for this patient who had a normally functioning pacemaker and who experienced 13 additional episodes of polymorphic ventricular tachycardia after his mild hypokalemia and hypomagnesemia were corrected.
This patient was initially treated with antiarrhythmic therapy until his ES trigger was resolved. Alternative management for ES includes catheter ablation. This method is more commonly used for patients who experience monomorphic VT during their ES, since this arrhythmia is often associated with a mechanism of scar-mediated reentry [4]. Additionally, surgical management options are emerging as potential protective therapies, including left cardiac sympathetic denervation for refractory ES patients who have failed maximal medical therapy and catheter ablation [4]. This patient demonstrated polymorphic VT and his ES had an identifiable trigger with successful targeted management, so catheter ablation and surgical intervention were not performed.
Clinical Implications and Future Directions
A 13% minority of ES patients have identifiable triggers for ES, which can include acute myocardial ischemia, electrolyte disturbances or sepsis [9]. This patient had a clear trigger of myocardial ischemia secondary to iatrogenic steal syndrome that resolved once his AV fistula was ligated. This case further demonstrates the importance of trigger identification and management for ES patients as he was ultimately able to discontinue antiarrhythmic therapy once his trigger had resolved.
Patients who develop ES have an increased mortality risk even compared to those with unclustered episodes of sustained ventricular arrhythmias [10]. They also have increased risk of worsening heart failure [3]. This patient’s ejection fraction had a clear reduction from his baseline 35-40% to 15-20% at the time of his ES. He also experienced multiple ICD shocks. Although patients with ES clearly experience an increased risk of mortality, the identification of ES as a risk factor for increased mortality remains controversial since other associated factors such as ICD shocks and worsening heart failure may also contribute to increased mortality [10]. Further research is needed to identify any potential causative link between ES and mortality risk.
Conclusion
CABGs and AV fistulas are both common vascular interventions against chronic disease. Understanding the effects that these procedures have on flow dynamics within the body is crucial to understanding their potential complications. In patients with CABG undergoing AV fistula creation, the arm contralateral to the LIMA should be selected for the fistula.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Acknowledgement
Acknowledge those who provided technical support during the study.
Financial Disclosure
No funding was not involved in the manuscript writing, editing, approval or decision to publish.
Data Availability
All authors had access to the data and a role in writing the manuscript. All data generated or analyzed in this study are included in this article. Access to data is possible with permission from the responsible author.
Consent for Publication
Informed consent was obtained from the patient for publication of this case report and is stated in the manuscript.
Author’s Contribution
All authors contributed equally for this paper.
References
- Kumar V, Abbas AK, Aster JC. Robbins basic pathology: robbins basic pathology E-Book. Ed. Elsevier Health Sci. 2017.
- Gaudino M, Serricchio M, Luciani N, Giungi S, Salica A, Pola R, et al. Risks of using internal thoracic artery grafts in patients in chronic hemodialysis via upper extremity arteriovenous fistula. Circulation. 2003;107(21):2653-5.
- Conti S, Pala S, Biagioli V, Del Giorno G, Zucchetti M, Russo E. Electrical storm: A clinical and electrophysiological overview. World J Cardiol. 2015;7(9):555-61.
- Gao D, Sapp JL. Electrical storm: definitions, clinical importance and treatment. Curr Opin Cardiol. 2013;28(1):72-9.
- Arya A, Haghjoo M, Dehghani MR. Prevalence and predictors of electrical storm in patients with implantable cardioverter-defibrillator. Am J Cardiol. 2006;97(3):389092.
- Pedersen CT, Kay GN, Kalman J, Borggrefe M, Della-Bella P, Dickfeld T, et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Europace. 2014;16(9):1257-83.
- Miller GA. Challenges and management of high-flow arteriovenous fistulae. Semin Nephrol. 2010; 32(6):545-50.
- Eifling M, Razavi M, Massumi A. The evaluation and management of electrical storm. Texas Heart Inst J. 2011;38(2):111.
- Elsokkari I, Sapp JL. Electrical storm: Prognosis and management. Prog Cardiovasc Dis. 2021;66:70-9.
- Guerra F, Shkoza M, Scappini L, Flori M, Capucci A. Role of electrical storm as a mortality and morbidity risk factor and its clinical predictors: a meta-analysis. Europace. 2014;16(3):347-53.
Author Info
Kiera Brigh Turner1





1Department of Internal Medicine, Orlando Regional Healthcare System, Orlando, Florida, USA
2Department of Internal Medicine, Wright State University Boonshoft School of Medicine, USA
3Department of Pediatrics, Wright State University Boonshoft School of Medicine, USA
4Charles E Schmidt College of Medicine, Florida Atlantic University, USA
5Delray Medical Center, Delray, Florida, USA
6Department of Academic Affairs and Research, USA
*Correspondence author: SJ Carlan, MD, Division of Academic Affairs and Research, USA; Email: stevecarlan@gmail.com
Copyright
Copyright© 2024 by Turner KB, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Citation: Turner KB, et al. Internal Mammary Artery Graft Flow Steal by a Large Dialysis Arteriovenous Fistula Characterized by Electrical Storm. Jour Clin Med Res. 2024;5(1):1-5. http://dx.doi.org/10.46889/JCMR.2024. 5108

