Case Report | Vol. 5, Issue 1 | Journal of Clinical Medical Research | Open Access |
Rare Case of Chronic Coxiella Endocarditis Without Fever or Classic Risk Factors
Brian Shaw1, Melinda Madden2, Antonio Crespo2, Ryan Shaw3, Steve J Carlan4*
1Department of Internal Medicine, USA
2Department of Infectious Disease, USA
3Biomedical Sciences, USA
4Division of Academic Affairs and Research, USA
*Correspondence author: Steve J Carlan, Division of Academic Affairs and Research, Orlando, Florida, USA; Email: stevecarlan@gmail.com
Citation: Shaw B, et al. Rare Case of Chronic Coxiella Endocarditis Without Fever or Classic Risk Factors. Jour Clin Med Res. 2024;5(1):1-5.
Copyright© 2024 by Carlan SJ, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
| Received 02 Jan, 2024 | Accepted 22 Jan, 2024 | Published 29 Jan, 2024 |
Abstract
Background: “Q-fever” is a zoonotic infectious disease caused by Coxiella burnetii which is most commonly transmitted globally through unpasteurized animal products or aerosolized fluid. This zoonosis is not thought to be common in developed countries due to modern utilization of pasteurization but risk for transmission remains high in those with extended contact with livestock and cattle. Acute Q-fever presents with an illness characterized by high fevers, myalgias, and segmental pneumonia, but rarely it may progress to chronic Q-fever. Chronic Q-fever most often presents with a culture-negative endocarditis with or without renal impairment and can be fatal if left untreated.
Case Report: In this case report, we present a 72-year-old male with a 3-month history of body pain, cough, and weight loss who was eventually discovered to have an afebrile presentation of Coxiella endocarditis. This patient was found to have a mitral valve vegetation diagnosed through transesophageal echocardiography and had positive serology for chronic Q-fever. Surprisingly, this patient had no classical exposures, no history of valvular dysfunction or prosthesis, and no clinical signs suggesting endocarditis, making his case markedly atypical. He was placed on a 12 -18-month course of antibiotics and was monitored on an outpatient basis.
Conclusion: Although chronic Q-fever is rare its variable presentation may cause diagnostic error if it is not kept on the differential. A transesophageal echocardiogram should be strongly preferred due to its superior visualization of heart valves. Serology remains the gold standard for diagnosis of Coxiella. When dealing with culture-negative endocarditis, clinicians should retain a high index of suspicion of Coxiella and obtain serologies for diagnosis.
Keywords: Q Fever; Endocarditis; Transesophageal Echocardiogram
Introduction
“Q-fever” is a zoonotic infectious disease caused by Coxiella burnetii, an obligate intracellular gram-negative coccobacillus [1]. Coxiella is often transmitted from animals to humans through ingestion of unpasteurized animal products or by inhalation of contaminated aerosols [1]. Infection is often asymptomatic in animals and humans, but symptomatic patients often present with an acute febrile illness characterized by high fevers, myalgias, and segmental pneumonia. [1,2]. Infections are not common in developed countries due to widespread pasteurization of animal products, but certain populations remain at high risk such as farmers, meat workers, and veterinarians due to their extended contact with cattle and livestock [3,4]. Seen in only 2% of cases, patients, patients may go on to develop chronic Q-fever which commonly presents with endocarditis with or without significant renal impairment. [2,5,6] Endocarditis in these patients is one of few which is considered “culture-negative”, as intracellular bacteria like Coxiella are notoriously difficult to culture in clinical setting [7]. In nearly all cases, endocarditis due to Q-fever presents in patients with pre-existing valvular disease or dysfunction [8,9]. Q-fever endocarditis is severe and can be fatal without quick diagnosis and treatment [8].
In this case, we present a 72-year-old male with an afebrile variant of chronic Q fever without any exposures to animals who presented with mitral valve endocarditis that lacked a previous history of valvular disease or dysfunction.
Case Report
A 72-year-old Haitian male with a past medical history of hypertension, type 2 diabetes mellitus, hyperlipidemia, and prostate cancer treated with radiation presented to the emergency room with a 3-month history of generalized body pain worse in his back, nonproductive cough, and 25lb weight loss. The patient had no novel exposures he could recall and denied alcohol or Intravenous (IV) drug abuse. The patient had no pets and no history of exposure to farm animals. The patient immigrated to the United States from Haiti 30 years prior and denied recent travel. The patient denied chest pain.
In the emergency room, the patient was afebrile and his vitals and physical exam were unremarkable. Initial laboratory investigation was remarkable only for elevated creatinine 1.5 mg/dL [0.7-1.3 mg/dL] and mildly decreased hemoglobin 12.3 g/dL [12.6-16.7 g/dL]. White blood cell count was within normal limits and remained so throughout his hospital course. Infectious workup including blood cultures was negative. EKG returned normal and his Chest X-Ray (CXR) revealed no evidence of disease. Urinalysis revealed cloudy urine with protein 100 mg/dL [Negative], blood ≥1 mg/dL [Negative], and red blood cell count >20/HPF [0-2/HPF]. Protein/creatinine ratio was elevated 0.5 [0.0-0.2], which further increased to 0.8 with elevated urine protein 41 [1-14mg/dL]. Urine electrolytes were unremarkable. The patient did not receive a kidney biopsy at that time. Due to his age and vague symptoms, atypical angina was considered and a stress test was ordered, which revealed mid-inferior and inferolateral defect in the region of the Right Coronary Artery (RCA). A Transthoracic Echocardiogram (TTE) revealed a normal Ejection Fraction (EF) of 65-69% and fibrocalcifications on the aortic valve. Left heart catheterization was planned by cardiology but was delayed due to steadily worsening renal function, interest in minimizing further kidney injury and contrast load, and lack of symptomatology or results indicating the urgency of invasive cardiac evaluation.
While waiting for medical optimization, the patient began experiencing increasing lightheadedness, and brain Magnetic Resonance Imaging (MRI) was obtained. Brain MRI revealed acute to subacute scattered punctate infarcts within both cerebellar hemispheres, left occipital, bilateral frontal, and bilateral parietal lobes highly suspicious for embolic phenomena. Surveillance for embolic sources was undertaken and a Transesophageal Echocardiogram (TEE) was performed showing EF of 55-59% and 1.3 cm x 0.8cm broad-based mass on the posterolateral mitral valve leaflet at the P1 scallop suspicious for infectious endocarditis, seen in Fig. 1,2.
Infectious disease was consulted, blood cultures were redrawn and the patient was started on empiric vacomycin and cefepime. Blood cultures remained negative and Human Immunodeficiency Virus (HIV), and Hepatitis B investigations were nonreactive. Vancomycin and cefepime were discontinued. The patient was noted to have poor dentition and panorex mandibular x-ray was obtained revealing questionable periapical lucency of the posterior left maxillary molar but no evidence of mandibular abscess or drainable lesion. Bartonella, Brucella, and Coxiella serologies were drawn. Q fever antibody screen was positive, titers were: 1:2048 for Phase I IgG [<1:16], 1:1024 for Phase II IgG [<1:16], Both Phase I and II IgM antibodies were <1:16. Antimicrobials were changed to doxycycline 100mg every 12 hours and hydroxychloroquine 200mg every 8 hours and was planned to continue for 18-24 months. Microbial cell-free DNA was obtained with the hope of isolating Coxiella, but ultimately resulted negative.
The patient was seen by cardiothoracic surgery and planned to undergo surgery for MV vegetation after coronary evaluation, however surgery was inevitably not indicated as the vegetation had decreased sufficiently in size. He has continued to follow up with infectious disease specialist’s outpatient for monitoring. No further embolisms occurred.
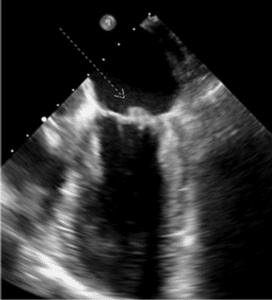
Figure 1: 1.3 cm x 0.8 cm broad based mass on the posterolateral mitral valve leaflet (white arrow) at the P1 scallop suspicious for vegetation visualized via transgastric short axis view from TEE imaging.

Figure 2: 1.3 cm x 0.8 cm broad based mass on the posterolateral mitral valve leaflet (arrow) at the P1 scallop suspicious for vegetation visualized via 3D post-processing based on TEE imaging
Discussion
Culture-negative endocarditis carries a broad differential diagnosis with many organisms that are difficult to isolate in cultures [10,11,12]. Traditional organisms include the “HACEK” organisms, Haemophilus species, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae [13]. Intracellular organisms such as Coxiella, Brucella, Bartonella and Trophyrema whipplei are diagnosed by serology [7,14,15].
Q-fever is often an asymptomatic illness, however symptomatic acute q-fever often presents with an acute febrile illness characterized by high fevers, myalgias and segmental pneumonia and symptomatic chronic Q-fever commonly presents with chronic fevers due to subactute endocarditis [1,2,5,6]. Fevers in chronic Q-fever are identical to those found typically in endocarditis [1,5,6]. Active Q-Fever infections are characterized by a fourfold increase in serum IgG between acute and convalescent samples or the presence of IgG and IgM antibodies directed against phase II organisms. In chronic Q Fever, the IgG response is most often directed against phase I organisms, resulting in phase I titers that are greater than phase II titers, as seen in our patient [16]. Instances of chronic infections of Q fever have been reported to present with endocarditis and vascular infections in only 2% of infected patients [2,5]. Additionally, as our patient exhibited, chronic Q fever may also present with kidney disease and renal failure by currently unclear mechanisms [6]. A two-week course of doxycycline is sufficient to treat acute Q-fever, however chronic Q-fever requires a combination of doxycycline and hydroxychloroquine for 18-24 months [17]. Culture-negative endocarditis already presents a substantial diagnostic challenge, but there are many aspects of this case that add to this dilemma. Firstly, our patient had no exposures that are traditionally associated with Q-fever. Traditional exposures include unpasteurized animal products, animal waste, or aerosolized fluid. Animals most associated with Coxiella include “large” animal livestock such as cows, goats, or sheep. Exposures to these animals are rare in developed countries such as the United States, with high risk-professions being associated with infection. Veterenarians, meat workers and farmers carry the highest risk of exposure due to their close proximity to animal waste, animal products and aerosolized animal fluid from births and other usual animal activity [3,4]. However, in a summary of case reports from 2000-2012 in the US, it was discovered that Coxiella is associated with exposure to cattle, goats, or sheep in only 61% of cases, emphasizing the importance of clinicians retaining a high index of suspicion even in cases without history of “classical” exposures [18]. Additionally, Coxiella endocarditis predominantly affects patients who have a history of underlying valvular damage, dysfunction, or implanted prosthetic valve, of which our patient lacks any history [8,9].
Secondly, our patient exhibited little to no symptoms of chronic Q-fever except for significant kidney injury of unclear etiology. Our patient was afebrile throughout his hospital courses and never reported fever or chills at any point, which is substantially atypical for Q-fever and is to our knowledge the first case in the literature to not be accompanied by either a low-grade or high-grade fever that is usually considered characteristic [1,2]. The patient also had no traditional symptoms of subacute endocarditis such as Roth spots, Osler nodes, Janeway lesions and splinter hemorrhages, which do not have high sensitivity [19,20]. Thirdly, initial TTE was negative which further decreased his suspicion for endocarditis. This highlights the position of TEE as the gold standard in the diagnosis of endocarditis due to superior visualization of valvular leaflets [21].
Conclusion
There are four main conclusions to draw from this case. First, though Coxiella is considered to be a disease seen mostly in developing countries or in high-risk populations such as farmers, meat handlers and veterinarians, in 39% of cases Coxiella infection occurred without identifiable exposures. Second, Chronic Q-fever may completely lack its characteristic fever or other signs of infection such as leukocytosis and may occur in patients whose valves are normal, contradicting previous thought. Kidney injury or renal failure may be the only presenting symptom in chronic Q-fever. Although chronic Q-fever is rare with only 2% of patients infected by Coxiella developing chronic infection, variable presentation may cause diagnostic error if it is not kept on the differential. Third, TTE is insufficient in the diagnosis of endocarditis and may delay diagnosis if negative. In cases with high clinical suspicion for endocarditis TEE should be strongly preferred due to its superior visualization of heart valves. TTE may be appropriate as initial evaluation depending on the clinical scenario as seen in our patient’s case. Fourth, serology remains the gold standard for diagnosis of Coxiella. When dealing with culture-negative endocarditis, clinicians should retain a high index of suspicion of Coxiella and obtain serologies for diagnosis. The findings from this case report are limited, this being a single case report and further study should continue.
Conflict of Interests
The authors have no conflict of interest to declare.
Declarations
All data generated or analyzed in this study are included in this article. Access to data is possible with permission from the responsible author.
References
- Knobel DL, Maina AN, Cutler SJ, et al. Coxiella burnetii in humans, domestic ruminants and ticks in rural western Kenya. Am J Trop Med Hyg. 2013;88(3):513-8.
- Wielders CC, Kampschreur LM, Schneeberger PM, et al. Early diagnosis and treatment of patients with symptomatic acute Q fever do not prohibit IgG antibody responses to Coxiella burnetii. Clin Vaccine Immunol. 2012;19(10):1661-6.
- Karakousis PC, Trucksis M, Dumler JS. Chronic Q fever in the United States. J Clin Microbiol. 2006;44(6):2283-7.
- Mori M, Roest HJ. Farming, Q fever and public health: agricultural practices and beyond. Arch Public Health. 2018;76:2.
- Abdali F, Hosseinzadeh S, Berizi E, Shams S. Prevalence of Coxiella burnetii in unpasteurized dairy products using nested PCR assay. Iran J Microbiol. 2018;10(4):220-6.
- Vacher-Coponat H, Dussol B, Raoult D, Casanova P, Berland Y. Proliferative glomerulonephritis revealing chronic Q fever. Am J Nephrol. 1996;16(2):159-61.
- Lambourne JR, Brooks T. Brucella and Coxiella; if you don’t look, you don’t find. Clin Med (Lond). 2015;15(1):91-2.
- Deyell MW, Chiu B, Ross DB, Alvarez N. Q fever endocarditis: a case report and review of the literature. Can J Cardiol. 2006;22(9):781-5.
- Sessa C, Vokrri L, Porcu P, Maurin M, Stahl JP, Magne JL. Abdominal aortic aneurysm and Coxiella burnetii infection: report of three cases and review of the literature. J Vasc Surg. 2005;42(1):153-8.
- Lin KP, Yeh TK, Chuang YC, Wang LA, Fu YC, Liu PY. Blood culture negative endocarditis: a review of laboratory diagnostic approaches. Int J Gen Med. 2023;16:317-27.
- Kanamoto T, Terakubo S, Nakashima H. Antimicrobial susceptibilities of oral isolates of Abiotrophia and Granulicatella according to the consensus guidelines for fastidious bacteria. Medicines (Basel). 2018;5(4):129.
- Brouqui P, Raoult D. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev. 2001;14(1):177-207.
- Sharara SL, Tayyar R, Kanafani ZA, Kanj SS. HACEK endocarditis: a review. Expert Rev Anti Infect Ther. 2016;14(6):539-45.
- Allizond V, Costa C, Sidoti F, et al. Serological and molecular detection of Bartonella henselae in specimens from patients with suspected cat scratch disease in Italy: A comparative study. PLoS One. 2019;14(2):e0211945
- Fenollar F, Amphoux B, Raoult D. A paradoxical Tropheryma whipplei western blot differentiates patients with whipple disease from asymptomatic carriers. Clin Infect Dis. 2009;49(5):717-23.
- Wielders CC, Boerman AW, Schimmer B, et al. Persistent high IgG phase I antibody levels against Coxiella burnetii among veterinarians compared to patients previously diagnosed with acute Q fever after three years of follow-up. PLoS One. 2015;10(1):e0116937.
- Kersh GJ. Antimicrobial therapies for Q fever. Expert Rev Anti Infect Ther. 2013;11(11):1207-14.
- Dahlgren FS, McQuiston JH, Massung RF, Anderson AD. Q fever in the United States: summary of case reports from two national surveillance systems, 2000-2012. Am J Trop Med Hyg. 2015;92(2):247-55.
- Ceglowska K, Nowomiejska K, Kiszka A, Koss MJ, Maciejewski R, Rejdak R. Bilateral macular roth spots as a manifestation of subacute endocarditis. Case Rep Ophthalmol Med. 2015;2015:493947.
- Chong Y, Han SJ, Rhee YJ, Kang SK, Yu JH, Na MH. Classic peripheral signs of subacute bacterial endocarditis. Korean J Thorac Cardiovasc Surg. 2016;49(5):408-12.
- Sordelli C, Fele N, Mocerino R, et al. Infective endocarditis: echocardiographic imaging and new imaging modalities. J Cardiovasc Echogr. 2019;29(4):149-55.
This work is licensed under a Creative Commons Attribution 2.0 International License.
Author Info
Brian Shaw1, Melinda Madden2, Antonio Crespo2, Ryan Shaw3, Steve J Carlan4*
1Department of Internal Medicine, USA
2Department of Infectious Disease, USA
3Biomedical Sciences, USA
4Division of Academic Affairs and Research, USA
*Correspondence author: Steve J Carlan, Division of Academic Affairs and Research, Orlando, Florida, USA; Email: stevecarlan@gmail.com
Copyright
Copyright© 2024 by Carlan SJ, et al. All rights reserved. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Citation: Shaw B, et al. Rare Case of Chronic Coxiella Endocarditis Without Fever or Classic Risk Factors. Jour Clin Med Res. 2024;5(1):1-5.

